global point of care
Independent Study Confirms Afinion™ 2 Delivers Lab-Quality
HbA1c Results at the Point of Care
Lab-accurate HbA1c Point of Care Testing
Accurate HbA1c testing is critical for diagnosing and managing diabetes. While many point-of-care (POC) devices are certified by the International Federation of Clinical Chemistry (IFCC) and the National Glycohemoglobin Standardization Program (NGSP), certification alone may not reflect
real-world performance.
A recent independent study evaluated 19 POC HbA1c devices, revealing significant variability in analytical quality. The Afinion™ 2 Analyzer emerged as a top performer, meeting or exceeding all key performance criteria.
Independent Study Overview: Comparing 19 HbA1c POC Devices
This study, conducted by Lenters-Westra, et al. evaluated the performance of 19 POC devices, including an assessment against the International Federation of Clinical Chemistry and Laboratory Medicine (IFCC) and NGSP certification criteria.1 The IFCC and NGSP organizations certify HbA1c assays to ensure accurate, standardized test results for reliable diagnosis and management of diabetes. The POC devices were compared to four laboratory reference methods used for manufacturer certification at the European Reference Lab Glycohemoglobin (ERL).
The study authors have previously conducted several performance evaluations of POC devices using established quality criteria, and the Afinion system has consistently performed well.2,3
The study was conducted in two phases, each evaluating 10 devices. The Afinion 2 was included in both phases and served as a benchmark to ensure consistency across the study. HbA1c concentrations were tested across the full diagnostic range, including healthy, prediabetic, and diabetic levels.
Key Findings: Afinion 2 Leads in HbA1c Testing Accuracy
- The Afinion 2 Analyzer achieved CVs ≤2.0% in NGSP units and CVs ≤3.0% in IFCC units. The Afinion 2 Analyzer achieved CVs ≤2.0% in NGSP units and CVs ≤3.0% in IFCC units at all concentration levels tested. Only two other devices met these criteria in NGSP units, and one other device in IFCC units at all concentration levels tested.
- Afinion 2 met IFCC and NGSP certification criteria. Annual certification may not be a sufficient indicator of quality as many of the POC devices were IFCC and/or NGSP certified but only five devices met both performance criteria when tested in this study.

- The Afinion 2 was the only device to achieve IFCC criteria indicating suitability for clinical trials. Ten POC devices met the minimum IFCC standard for routine clinical use (>2σ), while the Afinion 2 was the only device to achieve the more stringent IFCC criteria indicating suitability for use in clinical trials (>4σ).
- Analytical performance has important implications for clinical care. Some of the devices showed significant negative bias that could lead to misdiagnosis or incorrect management decisions
Detailed Results: HbA1c Device Performance Metrics
Precision
- Afinion 2, Atellica DCA, and OnCall MultiPro were the only three of the 19 POC devices evaluated that achieved CVs ≤2.0% calculated in NGSP units at all concentration levels tested (4.9%, 6.5%, and 9.0% HbA1c).
- Only Afinion 2 and Atellica DCA achieved CVs ≤3% calculated in IFCC/SI units at all three concentration levels tested (30, 48, and 75 mmol/mol).
Trueness (bias)
- Bias was calculated at three concentration levels: 5.0%, 6.5%, and 9.0%
HbA1c (30, 48, and 75 mmol/mol). - Afinion 2 demonstrated small bias against the mean of the four reference methods, ranging from -0.12 to -0.06% HbA1c (-1.3 to -0.7 mmol/mol).
IFCC Criteria
- The IFCC certification criteria requires the calculated sigma value to be >2σ for routine methods and >4σ for use in clinical trials. Sigma is calculated using the bias, CV, and a total allowable error (TAE) set at 6.9% (10% in SI units) using the formula: Sigma = (TAE – bias)/CV.
- The Afinion 2 was the only POC device to achieve the more stringent >4σ criterion. There were 10 devices that met the criteria for routine clinical use (>2σ).
NGSP Criteria
- The NGSP certification criteria requires 36 out of 40 results to be within ±5% of a laboratory reference method.
- The Afinion 2 and Quo-Lab were the only two POC devices to pass the NGSP criteria with all four of the laboratory reference methods in Phase 1. In Phase 2, the Afinion 2 passed the criteria with all but one reference method (Tosoh G11).
- Only five (Afinion 2, Cobas b101, Quo-Lab, Atellica DCA, On Call MultiPro) of the 19 POC devices passed the NGSP criteria with at least one of the laboratory reference methods.
High Concentration HbA1c Samples
- The study tested samples ranging from 11.6% (103 mmol/mol) to 15.7% HbA1c (148 mmol/mol). This is a useful evaluation, because external quality assessment (EQA) programs such as that administered by the College of American Pathologists (CAP) do not routinely evaluate samples with high HbA1c concentrations.4
- The Afinion 2 showed small differences in samples with high HbA1c concentrations when compared to the laboratory reference method (Tosoh G8), ranging from -2.1% to 2.3% difference.
Hemoglobin Variants
- The Afinion 2 could not be evaluated for hemoglobin variant interference, because the study used frozen specimens which cannot be run on the Afinion 2.
- However, it should be noted that the Afinion 2 has previously been shown to have no significant interference from hemoglobin variants, except with high concentrations (>10%) of HbF. The assay principle of the Afinion 2 (boronate affinity) is insensitive to hemoglobin variants.5
Summary of Key Performance Criteria
The table below summarizes the performance of all 19 POC devices against the criteria assessed in the study.
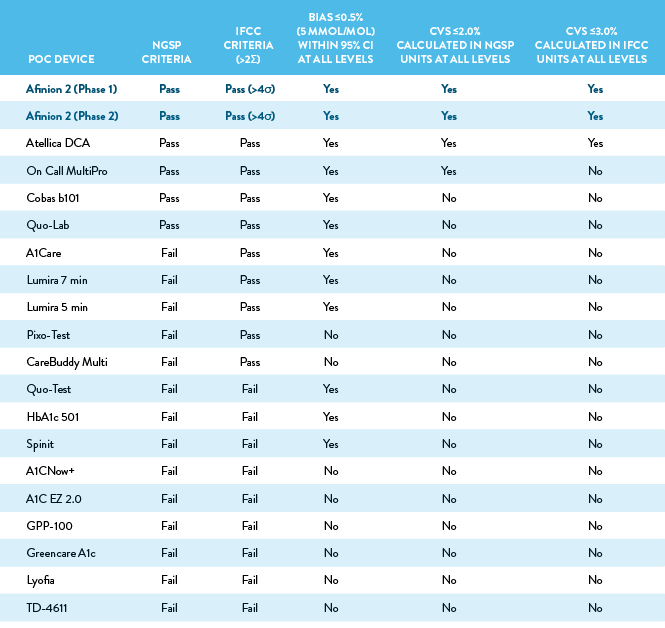
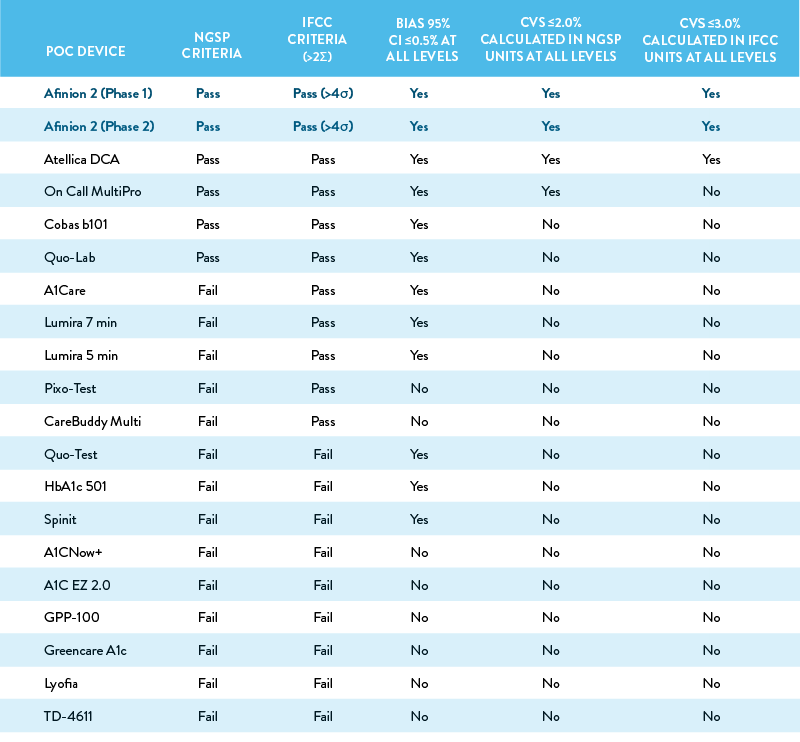

The NGSP certification criteria requires at least 36 of 40 samples to be within ±5% of the results from at least one laboratory reference method. The IFCC criteria is >2σ for routine methods and >4σ for use in clinical trials, as calculated by the formula: Sigma = (TAE – bias)/CV, where the TAE (total allowable error) is set at 6.9% (10% in SI units), bias is measured at 6.5% HbA1c (48 mmol/mol) per CLSI EP-9-A3, and CV is measured at 6.5 % HbA1c (48 mmol/mol) per CLSI EP-15-A3. Bias was evaluated at 3 concentration levels (5.0%, 6.5%, and 9.0% HbA1c, or 30, 48, and 75 mmol/mol). The study authors flagged a bias >0.5% (5 mmol/mol) within the 95% CI. Precision was evaluated at 3 concentration levels (4.9%, 6.5%, and 9.0% HbA1c, or 30, 48, and 75 mmol/mol); CVs >2.0% (>3.0%) were flagged by the study authors.
Evaluating Quality Beyond Certification
Although many of POC devices included in this evaluation are NGSP and IFCC certified, the majority performed poorly, indicating a single annual certification may not be a sufficient indicator of quality.
This independent evaluation highlights the variability in performance among NGSP and IFCC-certified POC HbA1c devices. While certification is important, it does not guarantee consistent analytical quality.
The Afinion 2 Analyzer stood out as the only device to meet all performance criteria, including the stringent >4σ IFCC threshold for clinical trials. These findings reinforce the importance of individual device validation and support the use of Afinion 2 in both routine and advanced clinical settings.
To explore the full dataset, methodology, and statistical analysis behind these findings, we encourage you to read the complete published study by Lenters-Westra et al.
References
- Lenters-Westra E, Singh P, Vetter B, English E. Challenges in Hb A1c Point-of-Care Testing: Only 5 of 19 Hb A1c Point-of-Care Devices Meet IFCC and NGSP Certification Criteria on Independent Evaluation. Clin Chem. Published online May 26, 2025. doi:10.1093/clinchem/hvaf059
- Lenters-Westra E, Slingerland RJ. Three of 7 hemoglobin A1c point-of-care instruments do not meet generally accepted analytical performance criteria. Clin Chem. 2014;60(8):1062–1072. doi:10.1373/clinchem.2014.224311
- Lenters-Westra E, English E. Evaluation of four HbA1c point-of-care devices using international quality targets: are they fit for the purpose? J Diabetes Sci Technol. 2018;12(4):762–770. doi:10.1177/1932296818785612
- National Glycohemoglobin Standardization Program (NGSP). College of American Pathologists (CAP) Survey Data. Available from: https://ngsp.org/CAPdata.asp.
- National Glycohemoglobin Standardization Program (NGSP). HbA1c assay interferences. Available from: https://ngsp.org/interf.asp.
- Kushner PR, Fonseca V, Nichols JH, Shubrook JH, Miller E, Wright E, DeFilippi C, Vassalotti JA. Clinical need for point-of-care testing for diabetes in Clinical and Laboratory Improvement Amendments–waived settings. Clin Diabetes. 2024; doi:10.2337/cd24-0071.



