Global Point Of Care
Aspectos destacados del punto de atención



Un nuevo estándar en sensibilidad con Determine™ HBsAg 2
La prueba de diagnóstico rápido más sensible del mundo que detecta el antígeno de superficie de la hepatitis B para su uso con suero, plasma o sangre completa.



Prueba BIOLINE™ HCV
Cuando la velocidad es importante, elegir una prueba de VHC rápida y de alta sensibilidad con un procedimiento de punción digital seguro puede marcar la diferencia.

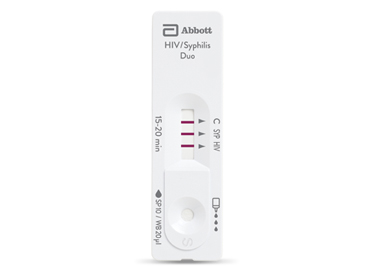
BIOLINE™ HIV/Syphilis Duo: Una prueba, dos resultados
Prueba y tratamiento en el mismo día para optimizar su programa de prevención de la transmisión maternoinfantil (PTMI).
Productos destacados
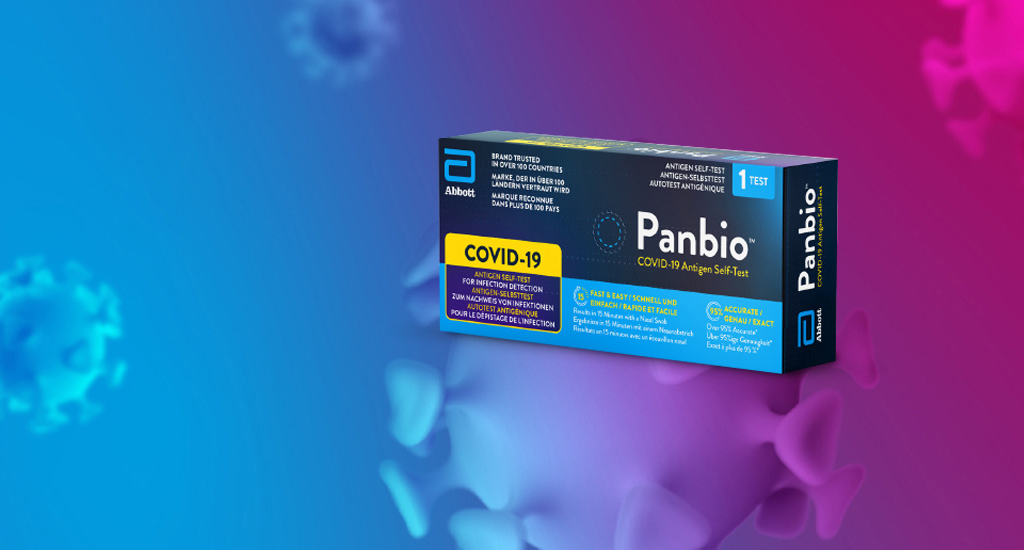
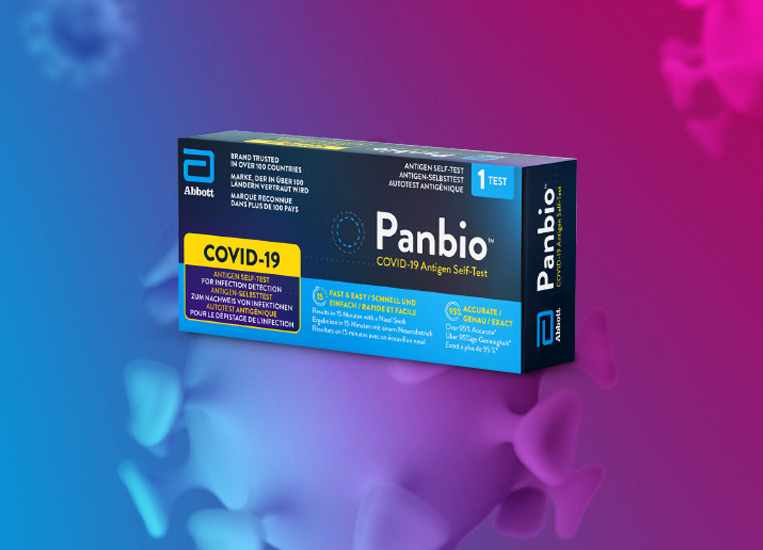
Panbio™ COVID-19 Ag Rapid Test Device
Para pacientes con sospecha de infección actual por COVID-19 No requiere instrumentación y proporciona resultados en 15 minutos, lo que lo convierte en una herramienta valiosa para pruebas masivas en entornos descentralizados.
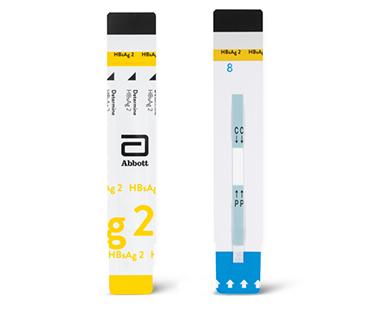
Determine™ HBsAg 2
Determine™ HBsAg 2, con una sensibilidad analítica de solo 0,1 UI/ml, es una prueba de flujo lateral rápida, fácil de usar y altamente sensible que permite una identificación exacta y rápida de pacientes HBsAg positivos, lo que aumenta la detección de casos de VHB y facilita la atención adecuada y rápida en cualquier entorno sanitario.
Bioline™ HIV/Syphilis Duo
Prueba en el mismo día y tratamiento para potenciar su programa de prevención de la transmisión maternoinfantil (PTMI)
Servicio. Asistencia. Respuestas.
Obtenga toda la asistencia técnica detallada y el servicio que necesita, justo de la forma requerida. Estamos a su disposición, en Internet y por teléfono.


Servicio. Asistencia. Respuestas.
Obtenga toda la asistencia técnica detallada y el servicio que necesita, justo de la forma requerida. Estamos a su disposición, en Internet y por teléfono.


Servicio. Asistencia. Respuestas.
Obtenga toda la asistencia técnica detallada y el servicio que necesita, justo de la forma requerida. Estamos a su disposición, en Internet y por teléfono.