GLOBAL POINT OF CARE
ID NOW™: When time matters
The ID NOW™ molecular respiratory testing platform can help you optimise resources and enhance patient care while maintaining control of costs.
For In Vitro Diagnostic Use Only
For In Vitro Diagnostic Use Only
ID NOW™: When time matters
The ID NOW™ molecular respiratory testing platform can help you optimise resources and enhance patient care while maintaining control of costs.




The ID Now Difference
Accurate, actionable results during the first patient encounter*
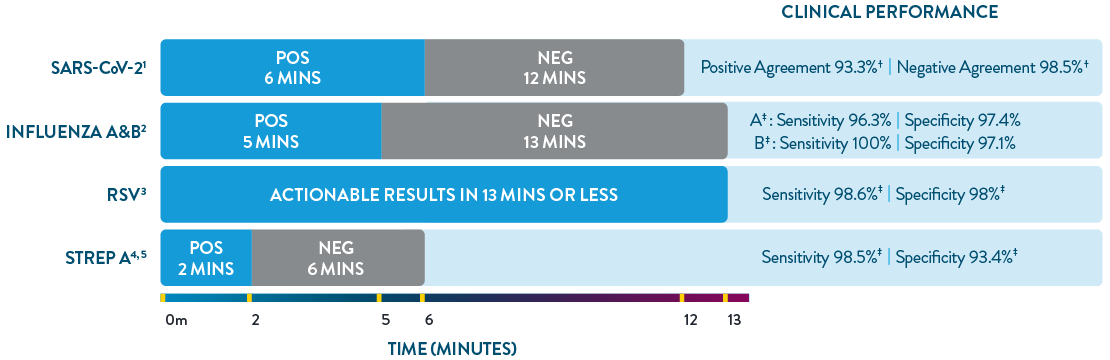
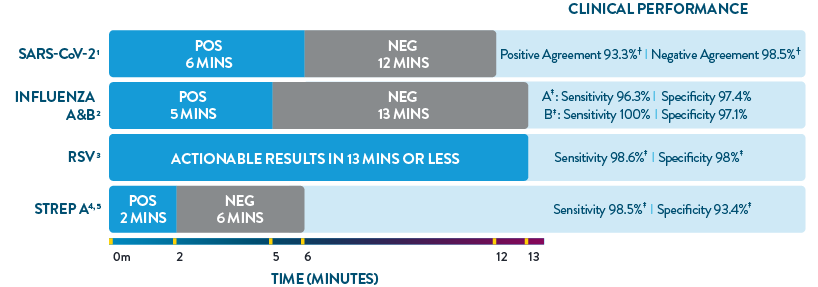
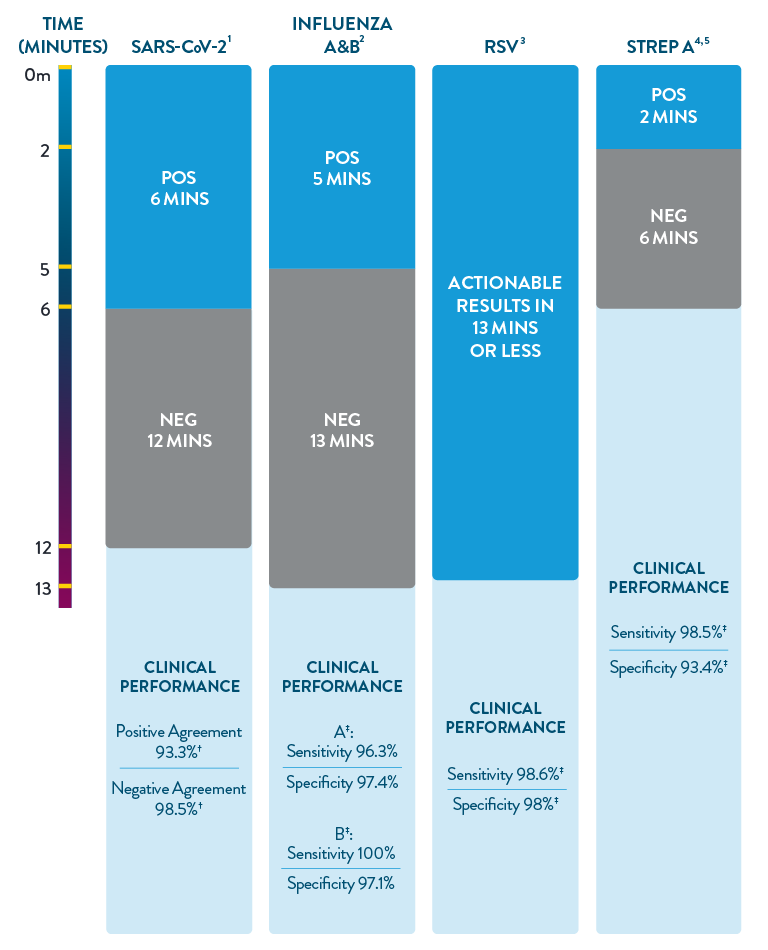
ID NOW™ is the only POC molecular testing platform to provide results in 13 minutes or less for SARS-CoV-2, Influenza A & B, RSV, and Strep A. With accuracy equivalent to RT-PCR and superior to rapid antigen-based tests, ID NOW™ extends diagnostic resources beyond the central lab to the actual sites of patient care, so clinicians can start the right treatment sooner.
The point-of-care platform that improves your processes
ID NOW™ integrates seamlessly into acute care settings and gives your lab increased capacity and improved efficiency. By reducing delays, shortening turnaround times, and freeing up personnel and resources for other essential diagnostics, ID NOW™ can help reduce strain on hospital utilities and resources.
Watch the video to explore how id now™ can optimise your respiratory testing pathway at the point of care.
Watch Dr. Yusuf Gray, Advanced Biomedical Scientist, discuss his experience with ID NOW™ in the ED.
The molecular testing platform that gives clinicians a choice
The ID NOW™ The ID NOW platform allows clinicians the ability to choose a specific assay based on the clinical presentation of the patient circulating prevalence, and seasonality of SARS-CoV-2, Influenza A & B, RSV, and Strep A. Selecting the right assay at the right time helps optimise positive predictive value, reduce unnecessary testing, and enhance diagnostic stewardship.
WHY CONSIDER DISEASE SEASONALITY AND PREVALENCE?
Because they impact the positive predictive value (PPV) of the assay—the likelihood that a person with a positive test result actually has the disease being tested.
Imagine testing 100 patients for the flu using a hypothetical diagnostic test with a sensitivity of 100% and a specificity of 97%.
During peak flu season, there will be many positive test results and the PPV will be high.
In non-peak season there will be fewer positive test results and the PPV will be much
lower—in fact, as many as half of all positive results are likely to be false positive.
Multiplex respiratory panels are known to likely produce false-positive results when pathogen prevalence is low, using a focused single-target molecular platform such as
ID NOW™ can help reduce such inconsistent or false-positive respiratory results.6
Learn more about diagnostic stewardship and positive
predictive value.
PPV During
High Prevalence

Low Prevalence



*76% of emergency ED clinicians spend less than 16 minutes with a patient.
† Within 7 days of symptom onset.
‡ Results are specific to direct swab.
ED: emergency department; pED: pediatric emergency department; EMR: electronic medical record; POC: point of care; PPV: positive predictive value; RT-PCR: reverse transcription polymerase chain reaction; UTM: universal transport medium; VTM: viral transport medium.
- ID NOW™ COVID-19 2.0 Product Insert.
- ID NOW™ Influenza A & B 2 Product Insert.
- ID NOW™ RSV Package Insert. Abbott Diagnostics Scarborough, Inc.; 2020. https://imgcdn.mckesson.com/CumulusWeb/Click_and_learn/ID_Now_RSV_Package_Insert.pdf.
- ID NOW™ Strep A 2 Product Insert.
- Abbott. Data on File. ID NOW™ TM Strep A 2 clinical assertion data.
- U.S. FDA. 21 CFR 866.4001 – Multiplex respiratory viral and bacterial nucleic acid detection assay – special controls section describing higher likelihood of false positives at low prevalence.



