Global point of care
SELECT A PRODUCT, VAS/eVAS TYPE, AND SOFTWARE VERSION FROM THE DROP-DOWN MENUS.
VALUE ASSIGNMENT SHEETS
Use Value Assignment Sheets (VAS) to locate the correct target values and ranges for your i-STAT System test cartridge controls and calibration verification materials. You may also access electronic Value Assignment Sheets (eVAS).
i-STAT 1 content updated 13-January-2026
To find the correct VAS and ranges, you need to:
- Locate the cartridge type and lot number found on your cartridge box or pouch.
- Choose the corresponding lot and type from the menu below.
- Identify the corresponding cartridge type and lot prefix letter WITHIN the value assignment sheet.

CLEW A51 EXPIRES 17 JUNE 2026
ACT LEVEL 1 CONTROL
ACT LEVEL 2 CONTROL
β-Hcg Calibration Verification Set
β-HCG LEVEL 1 CONTROL
β-HCG LEVEL 2 CONTROL
β-HCG LEVEL 3 CONTROL
BNP Calibration Verification Set
BNP LEVEL 1 CONTROL
BNP LEVEL 2 CONTROL
BNP LEVEL 3 CONTROL
CHEM8+ CALIBRATION VERIFICATION LEVEL 1B
CK-MB Calibration Verification Set
CK-MB LEVEL 1 CONTROL
CK-MB LEVEL 2 CONTROL
CK-MB LEVEL 3 CONTROL
Ctni Calibration Verification Set
CTNI LEVEL 1 CONTROL
CTNI LEVEL 2 CONTROL
CTNI LEVEL 3 CONTROL
i-STAT CALIBRATION VERIFICATION SET
i-STAT LEVEL 1 CONTROL
i-STAT LEVEL 2 CONTROL
i-STAT LEVEL 3 CONTROL
PT LEVEL 1 CONTROL
PT LEVEL 2 CONTROL
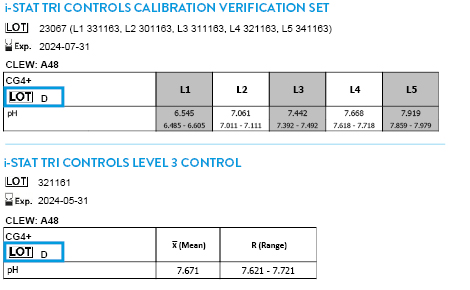
Tricontrols Calibration Verification Set
TRICONTROLS LEVEL 1 CONTROL
TRICONTROLS LEVEL 2 CONTROL
TRICONTROLS LEVEL 3 CONTROL
Additional Resources
ELECTRONIC VALUE ASSIGNMENT SHEETS
You can automatically update your device(s) with the latest Value Assignment Sheet data to streamline and simplify the liquid quality control process.
i-STAT 1 DOWNLOAD INSTRUCTIONS
- Select the file for download and Save. Do not rename the file.
- Confirm “Save as type” is either:
- .VAS Document
- VAS File
- All Files
FOR DE CUSTOMERS:
- Save the file to any directory accessible to the i-STAT/DE and click “Save”.
- Close the “Download Complete” window when finished.
- Access the DE Customization workspace:
Upload the eVAS file to i-STAT/DE:
- Click Update i-STAT/DE at the top of the Customization Workspace and select Upload Update File.
- Click Browse when the “Specify file for i-STAT/DE update” box opens.
- Navigate to the directory location where the eVAS file was saved.
- Select the eVAS file and click Open.
- Click “Upload.” A confirmation message will appear if the upload is successful.
i-STAT 1 content updated 13-January-2026









