Global point of care
ID NOW™: When time matters
The ID NOW™ molecular respiratory testing platform delivers fast, reliable results, enabling you to expand your lab’s impact, reach, and efficiency while maintaining control of critical diagnostics
For In Vitro Diagnostic Use Only
For In Vitro Diagnostic Use Only
ID NOW™: When time matters
The ID NOW™ molecular respiratory testing platform delivers fast, reliable results, enabling you to expand your lab’s impact, reach, and efficiency while maintaining control of critical diagnostics




The ID Now Difference
Accurate, actionable results during the first patient encounter*
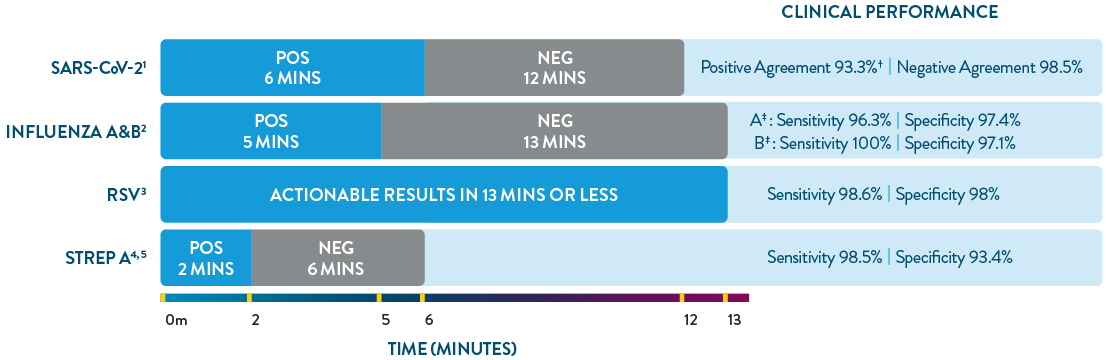
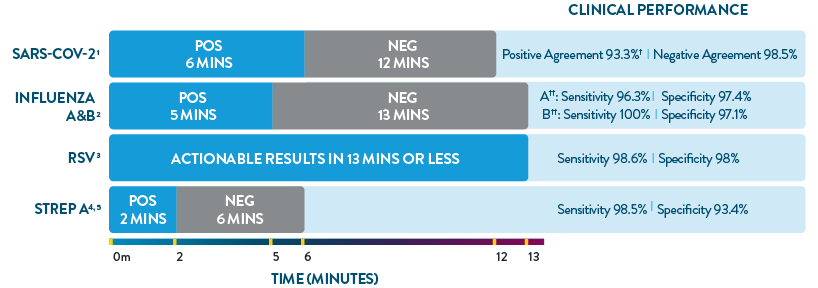
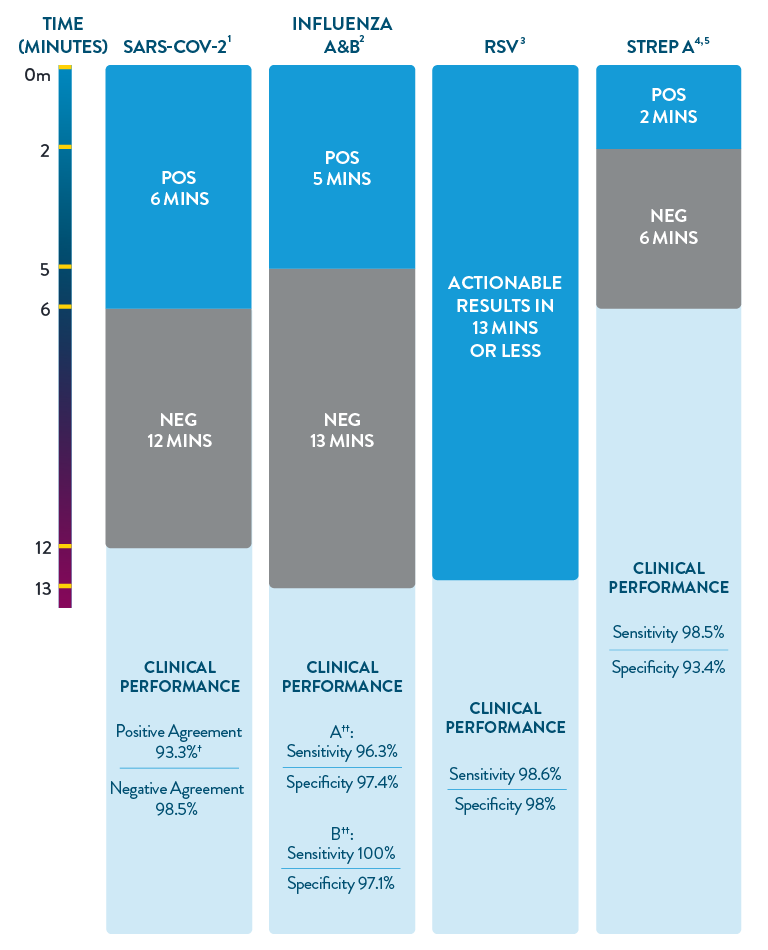
ID NOW™ is the only POC molecular testing platform to provide results in 13 minutes or less for SARS-CoV-2, Influenza A & B, RSV, and Strep A. With accuracy equivalent to RT-PCR and superior to rapid antigen-based tests, ID NOW™ extends your diagnostic resources beyond the central lab to the actual sites of patient care, so clinicians can start the right treatment sooner.
The point-of-care platform that improves your processes
ID NOW™ integrates seamlessly into acute care settings. By reducing delays, shortening turnaround times, and freeing up personnel and resources for other essential diagnostics, ID NOW™ can enhance your lab’s testing capacity and help reduce strain on hospital utilities and resources.
Watch the video to explore how id now™ can optimise your respiratory testing pathway at the point of care.
Watch Dr. Yusuf Gray, Advanced Biomedical Scientist, discuss his experience with ID NOW™ in the ED.
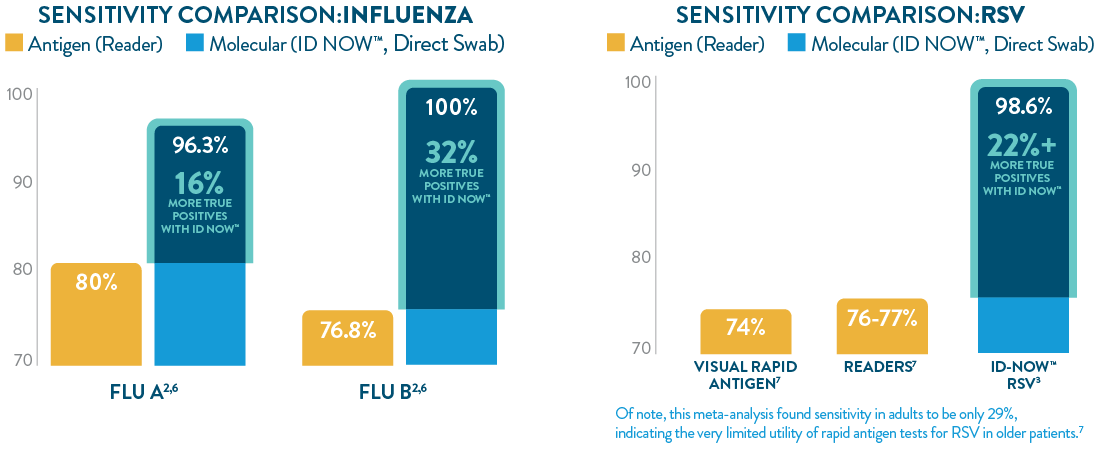
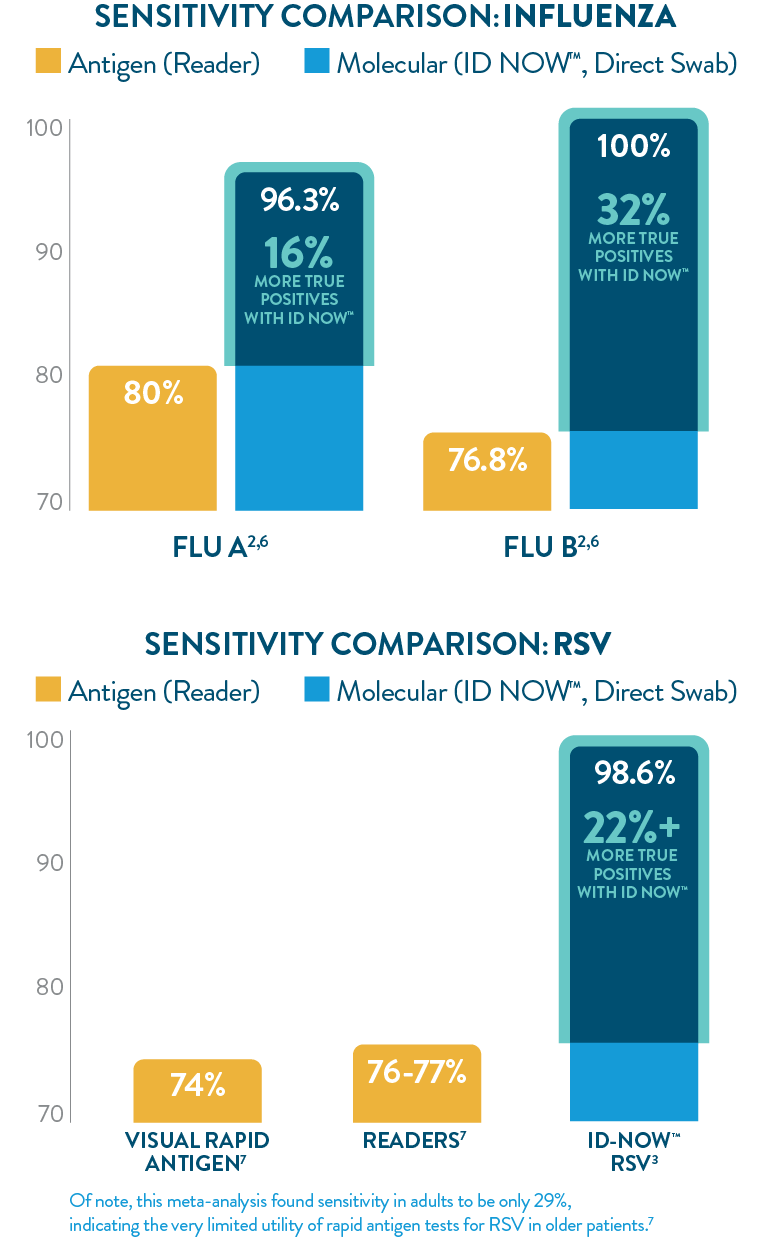
ID NOW™ offers greater sensitivity than POC LFA or FIA
The ID NOW™ platform’s isothermal NEAR technology utilizes proprietary enzymes and constant temperatures to provide rapid results with superior sensitivity. ID NOW™ detects more true positives for SARS-CoV-2, Influenza A & B, and RSV than rapid antigen tests,1-3,6,7 and eliminates the need for additional culture confirmation of Strep A,5 further reducing the burden on your lab.
*76% of emergency ED clinicians spend less than 16 minutes with a patient.8
† Within 7 days of symptom onset.
‡ Results are specific to direct swab.
ED: emergency department; pED: pediatric emergency department; EMR: electronic medical record; FIA: fluorescence immunoassay; LFA: lateral flow assay; POC: point of care; PPV: positive predictive value; RT-PCR: reverse transcription polymerase chain reaction; UTM: universal transport medium; VTM: viral transport medium.
- ID NOW™ COVID-19 2.0 Product Insert.
- ID NOW™ Influenza A & B 2 Product Insert.
- ID NOW™ RSV Package Insert. Abbott Diagnostics Scarborough, Inc.; 2020. https://imgcdn.mckesson.com/CumulusWeb/Click_and_learn/ID_Now_RSV_Package_Insert.pdf.
- ID NOW™ Strep A 2 Product Insert.
- Abbott. Data on File. ID NOW™ TM Strep A 2 clinical assertion data.
- Merckx J, Wali R, Schiller I, et al. Diagnostic accuracy of novel and traditional rapid tests for influenza infection compared with reverse transcriptase polymerase chainreaction: A systematic review and meta-analysis. Ann Intern Med. 2017;167(6):394-409.
- Chartrand, C. et al. Diagnostic accuracy of rapid antigen detection tests for respiratory syncytial virus infection: Systematic review and meta-analysis. J Clin Microbiol.2015;53(12):3738-3749.
- Grisham S. Medscape Emergency Medicine Physician—Physician Compensation Report 2017. Accessed December 18, 2024.https://www.medscape.com/slideshow/compensation-2017-emergency-medicine-6008568.



