Screen saver
The screen saver will turn on after 3 minutes if the touch screen is not in use. To reactivate, touch the screen.
Light signals (the red and green LEDs)
The red diode is illuminated when the analyzer is busy. A flashing red light is seen when an information code is displayed. The green diode is illuminated when the analyzer is ready for use. A flashing green light indicates completion of an analysis.
Sound signals
A short beep indicates completion of an analysis. Two beeps mean that an information code or message is displayed.
Calibration
The Afinion™ 2 Analyzer has been manufactured to deliver reliable and accurate results. During manufacturing, the analyzers are calibrated against a reference system. This procedure has been established to ensure that all analyzers operate within identical tolerance limits.
Test specific calibration data are established for each lot of test cartridges and then stored in the barcode label. When the test cartridge enters the analyzer, the integrated camera reads the barcode. The calibration data for the actual lot are transferred to the instrument and used for calculating the results. Calibration by the operator is thus not required.
Cleaning and maintenance
No maintenance of the Afinion™ 2 Analyzer is required other than cleaning the exterior and cartridge chamber.
Cleaning the exterior of the Afinion™ 2 Analyzer should be performed whenever necessary.
The cartridge chamber should be cleaned immediately if materials or liquids are spilled in the cartridge chamber. For regular maintenance (removal of dust particles etc.), the cartridge chamber should be cleaned every 30 days.
Please refer to the user manual for complete instructions.






 ON/OFF button: Turns the power to the Analyser on and off.
ON/OFF button: Turns the power to the Analyser on and off. Red and Green LEDs: Light emitting diodes (LEDs) that indicates whether the Analyser is busy or not.
Red and Green LEDs: Light emitting diodes (LEDs) that indicates whether the Analyser is busy or not. Touch screen: Allows you to communicate with the Analyser through touch buttons and messages.
Touch screen: Allows you to communicate with the Analyser through touch buttons and messages. The lid: Covers and protects the cartridge chamber.
The lid: Covers and protects the cartridge chamber. Ethernet port for connection to LIS/HIS/EMR systems. Use shielded cable.
Ethernet port for connection to LIS/HIS/EMR systems. Use shielded cable. USB-A: Connectors for printer, USB flash and barcode reader.
USB-A: Connectors for printer, USB flash and barcode reader. Power input for power supply connection.
Power input for power supply connection.





 to enter Main Menu
to enter Main Menu
 to enter Main Menu
to enter Main Menu
 in the configuration menu to enter the patient ID on/off option.
in the configuration menu to enter the patient ID on/off option. to disable the operator ID function.
to disable the operator ID function. to enable the patient ID function.
to enable the patient ID function. to accept and return to the configuration menu.
to accept and return to the configuration menu.
 in the configuration menu to enter the operator configuration menu.
in the configuration menu to enter the operator configuration menu.
 in the configuration menu to enter the operator configuration menu.
in the configuration menu to enter the operator configuration menu. to disable the operator ID function.
to disable the operator ID function. to enable operator ID. Any operator ID is accepted.
to enable operator ID. Any operator ID is accepted. to enable operator ID with verification.
to enable operator ID with verification.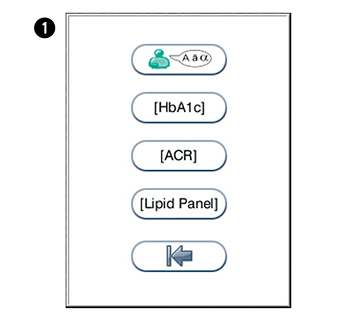
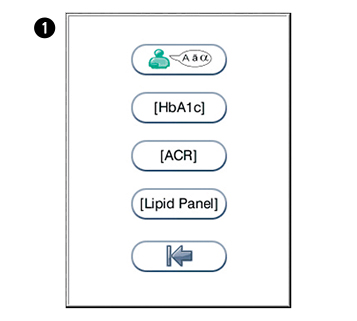
 in the configuration menu to enter the regional settings menu.
in the configuration menu to enter the regional settings menu. to enter the language selection. Touch the arrow in the window to view other options. N.B English is the default language.
to enter the language selection. Touch the arrow in the window to view other options. N.B English is the default language. to enter HbA1c units. Touch the arrow in the window to view other options. Options: mmol/moL (UK), %, eAG mmol/L (estimated glucose average).
to enter HbA1c units. Touch the arrow in the window to view other options. Options: mmol/moL (UK), %, eAG mmol/L (estimated glucose average). to enter ACR units. Touch the arrow in the window to view other options. Options: mg/mmol (UK), mg/g.
to enter ACR units. Touch the arrow in the window to view other options. Options: mg/mmol (UK), mg/g. to enter lipid panel configuration menu. Touch the arrow in the window to view other options. Options: mmol/L (UK), mg/dL.
to enter lipid panel configuration menu. Touch the arrow in the window to view other options. Options: mmol/L (UK), mg/dL.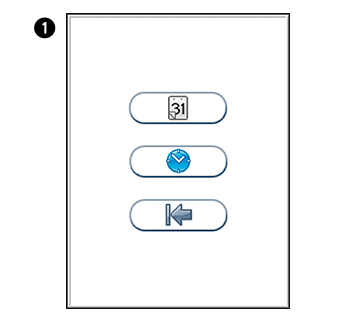
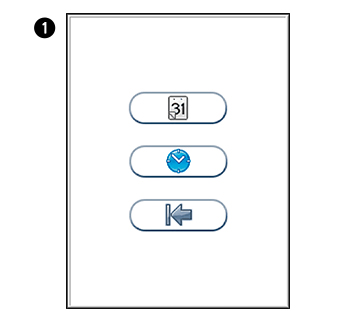
 in the Configuration menu to enter Date/time menu.
in the Configuration menu to enter Date/time menu. to enter Date setting.
to enter Date setting. to enter Time setting.
to enter Time setting.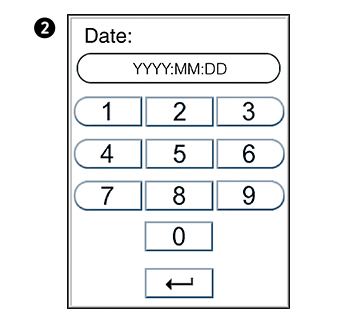
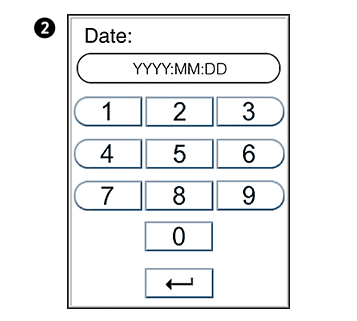
 to confirm and return to previous view.
to confirm and return to previous view.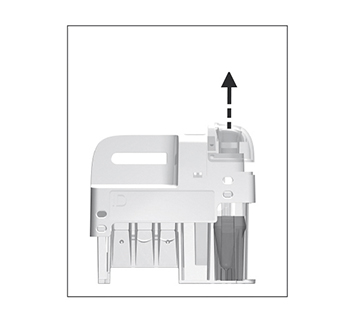
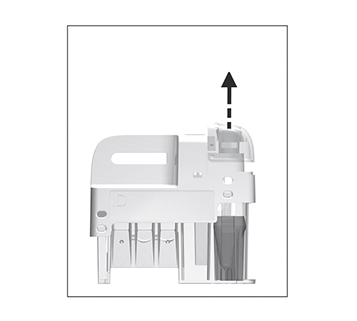
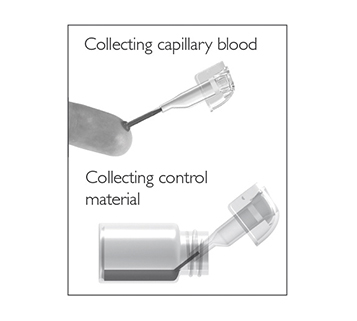
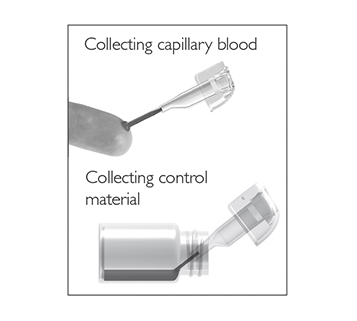
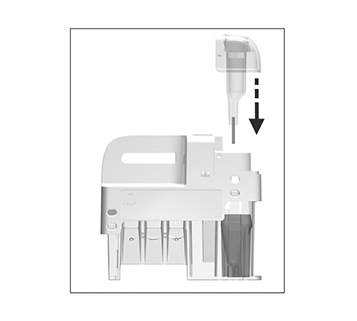
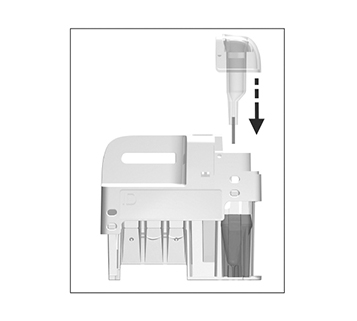


 for patient samples, or touch
for patient samples, or touch  for controls. The lid opens automatically.
for controls. The lid opens automatically. 








