Global Point Of Care
Determine™ HIV Early Detect
FAST4WARD DETECCIÓN RÁPIDA DE LA INFECCIÓN AGUDA POR VIH.
Determine™ HIV Early Detect es la primera y única prueba de diagnóstico rápido (RDT) de 4.ª generación precalificada por la OMS.
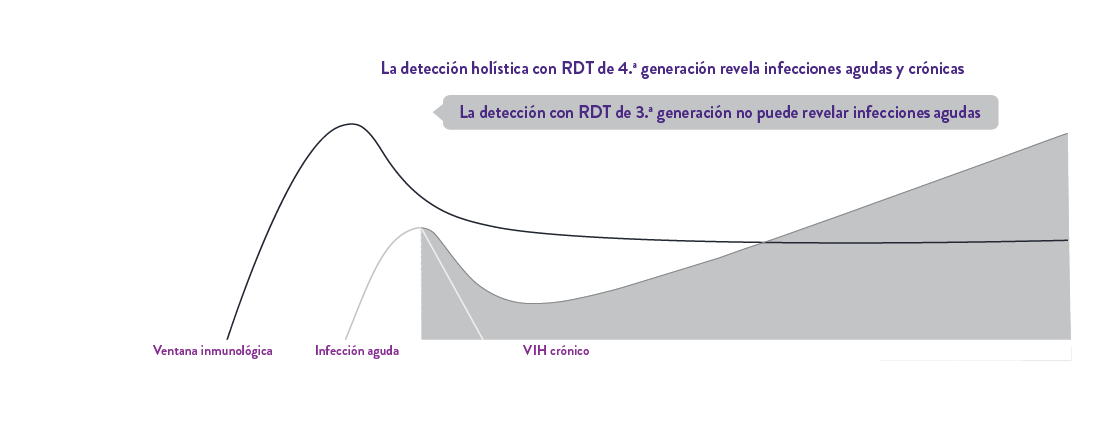
Determine™ HIV Early Detect de 4.ª generación establece un nuevo estándar en las pruebas de detección del VIH en el punto de atención con su excepcional doble precisión para un enfoque holístico de detección de infecciones agudas y crónicas por el VIH, protegiendo a pacientes y comunidades.
Determine™ HIV Early Detect proporciona una prueba sencilla y resultados exactos en tan solo 20 minutos, utilizando sangre completa obtenida por punción digital o venopunción, o suero/plasma.
Producto no disponible en todos los mercados.

Beneficios
DOBLE PRECISIÓN EXCEPCIONAL
Detecta con precisión la infección aguda por VIH con el antígeno p241 y la infección crónica por VIH con su mayor sensibilidad analítica de anticuerpos1, lo que la convierte en la prueba rápida de autoanálisis ideal.
IMPACTO CLÍNICO Y ECONÓMICO SIGNIFICATIVO
Detiene la transmisión y mejora el pronóstico con un valor clínico y económico significativo a través de la detección y el tratamiento del VIH agudo.2 Proporciona un valor económico significativo al reducir los costes asociados a las nuevas transmisiones y los diagnósticos tardíos.3,4
PRUEBAS SENCILLAS PARA EL CUIDADO DEL PACIENTE
Procedimiento de prueba sencillo que mejora la experiencia del usuario y la facilidad de uso mediante un flujo de trabajo de prueba flexible y una ventana de tiempo de lectura ampliada.1
Determine™ HIV Early Detect tiene una doble precisión EXCEPCIONAL para detectar infecciones agudas y crónicas para un enfoque holístico de detección.


Documentos úteis
Documentos útiles
Especificaciones
- Sensibilidad del 100 % y especificidad del 99,72 %
* Consulte en la hoja informativa del producto la sensibilidad analítica al antígeno
p24 del VIH-1 - Método: Flujo lateral
- Tiempo para los resultados: 20 minutos
- Almacenamiento: De 2 a 30 °C
- Volumen de la muestra: 50 μl
- Periodo de validez de la prueba: 18 meses
- Tipos de muestra: Suero/plasma, sangre completa obtenida por punción digital y por venipunción
CÓDIGO DEL PRODUCTO
| Determine™ HIV Early Detect | Con marcado CE | Sin marcado CE |
|---|---|---|
| Determine™ HIV Early Detect 20 Test Kit | 7D2846 | 7D2842 |
| Determine™ HIV Early Detect 100 Test Kit | 7D2847 | 7D2843 |
| Determine™ HIV Early Detect SET (100 pruebas y accesorios)* | 7D2843SET | |
| Chase Buffer (para 100 pruebas) | 7D2243 | 7D2243 |
| Determine™ HIV Controls (3 pos, 1 neg)* | 7D2253 | |
| EDTA Capillary Tubes (100) | 7D2227 | 7D2222 |
* Uso en investigación solamente en Australia
Vídeos

Procedimiento de análisis de sangre completa por venopunción

Procedimiento de análisis de suero y plasma
Productos relacionados
Ver referencias
- WHO Prequalification of In Vitro Diagnostics. (2020). Public report: Product: Determine™ HIV Early Detect (PQDx-0243-013-00). World Health Organization. https://extranet.who.int/prequal/sites/default/files/whopr_files/PQDx_0243-013-00DetermineHIVEarlyDetect_v8.pdf
- Kroon EDMB, Phanuphak N, Shattock AJ, et al. Acute HIV infection detection and immediate treatment estimated to reduce transmission by 89% among men who have sex with men in Bangkok. J Int AIDS Soc. 2017;20(1):21708. doi:10.7448/IAS.20.1.21708
- Tran H, Saleem K, Lim M, et al. Global estimates for the lifetime cost of managing HIV. AIDS. 2021;35(8):1273-1281. https://doi.org/10.1097/qad.0000000000002887
- Fleishman JA, Yehia BR, Moore RD, Gebo KA; HIV Research Network. The economic burden of late entry into medical care for patients with HIV infection. Med Care. 2010;48(12):1071-1079. https://doi.org/10.1097/mlr.0b013e3181f81c4a
- Patel P, Bennett B, Sullivan T, Parker MM, Heffelfinger JD, Sullivan PS; CDC AHI Study Group. Rapid HIV screening: missed opportunities for HIV diagnosis and prevention. J Clin Virol. 2012 May;54(1):42-7. https://doi.org/10.1016/j.jcv.2012.01.022



