Global Point of Care
SELECT A PRODUCT, VAS/eVAS TYPE, AND SOFTWARE VERSION FROM THE DROP-DOWN MENUS.
VALUE ASSIGNMENT SHEETS
Use Value Assignment Sheets (VAS) to locate the correct target values and ranges for your i-STAT System test cartridge controls and calibration verification materials. You may also access electronic Value Assignment Sheets (eVAS).
i-STAT 1 content updated 13-January-2026
To find the correct VAS and ranges, you need to:
- Locate the cartridge type and lot number found on your cartridge box or pouch.
- Choose the corresponding lot and type from the menu below.
- Identify the corresponding cartridge type and lot prefix letter WITHIN the value assignment sheet.
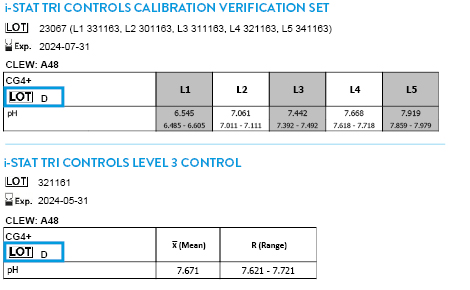

CLEW A51 EXPIRES 17 JUNE 2026
ACT LEVEL 1 CONTROL
ACT LEVEL 2 CONTROL
β-Hcg Calibration Verification Set
β-HCG LEVEL 1 CONTROL
β-HCG LEVEL 2 CONTROL
β-HCG LEVEL 3 CONTROL
BNP Calibration Verification Set
BNP LEVEL 1 CONTROL
BNP LEVEL 2 CONTROL
BNP LEVEL 3 CONTROL
CHEM8+ CALIBRATION VERIFICATION LEVEL 1B
CK-MB Calibration Verification Set
CK-MB LEVEL 1 CONTROL
CK-MB LEVEL 2 CONTROL
CK-MB LEVEL 3 CONTROL
Ctni Calibration Verification Set
CTNI LEVEL 1 CONTROL
CTNI LEVEL 2 CONTROL
CTNI LEVEL 3 CONTROL
hs-TnI Calibration Verification Set
hs-TnI LEVEL 1 CONTROL
hs-TnI LEVEL 2 CONTROL
hs-TnI LEVEL 3 CONTROL
i-STAT CALIBRATION VERIFICATION SET
i-STAT LEVEL 1 CONTROL
i-STAT LEVEL 2 CONTROL
i-STAT LEVEL 3 CONTROL
PTplus LEVEL 1 CONTROL
PTplus LEVEL 2 CONTROL
PT LEVEL 1 CONTROL
PT LEVEL 2 CONTROL
Tricontrols Calibration Verification Set
TRICONTROLS LEVEL 1 CONTROL
TRICONTROLS LEVEL 2 CONTROL
TRICONTROLS LEVEL 3 CONTROL
Additional Resources
ELECTRONIC VALUE ASSIGNMENT SHEETS
You can automatically update your device(s) with the latest Value Assignment Sheet data to streamline and simplify the liquid quality control process.
i-STAT 1 DOWNLOAD INSTRUCTIONS
- Select the file for download and Save. Do not rename the file.
- Confirm “Save as type” is either:
- .VAS Document
- VAS File
- All Files
FOR DE CUSTOMERS:
- Save the file to any directory accessible to the i-STAT/DE and click “Save”.
- Close the “Download Complete” window when finished.
- Access the DE Customization workspace:
Upload the eVAS file to i-STAT/DE:
- Click Update i-STAT/DE at the top of the Customization Workspace and select Upload Update File.
- Click Browse when the “Specify file for i-STAT/DE update” box opens.
- Navigate to the directory location where the eVAS file was saved.
- Select the eVAS file and click Open.
- Click “Upload.” A confirmation message will appear if the upload is successful.
i-STAT 1 content updated 13-January-2026
eVAS File For Download
Additional Resources
VALUE ASSIGNMENT SHEETS
Use Value Assignment Sheets (VAS) to locate the correct target values and ranges for your i-STAT System test cartridge controls and calibration verification materials. You may also access electronic Value Assignment Sheets (eVAS).
i-STAT Alinity content updated 13-January-2026
To find the correct VAS and ranges, you need to:
- Locate the cartridge type and lot number found on your cartridge box or pouch.
- Choose the corresponding lot and type from the menu below.
- Identify the corresponding cartridge type and lot prefix letter WITHIN the value assignment sheet.
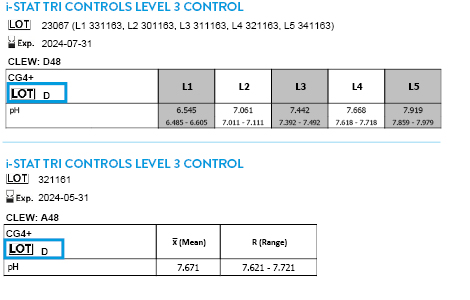

CLEW D50 EXPIRES 14 JANUARY 2026
ACT LEVEL 1 CONTROL
ACT LEVEL 2 CONTROL
CHEM8+ CALIBRATION VERIFICATION LEVEL 1B
hs-TnI Calibration Verification Set
hs-TnI LEVEL 1 CONTROL
hs-TnI LEVEL 2 CONTROL
hs-TnI LEVEL 3 CONTROL
i-STAT calibration verification set
i-STAT LEVEL 1 CONTROL
i-STAT LEVEL 2 CONTROL
i-STAT LEVEL 3 CONTROL
TBI Calibration Verification Set
TBI Level 1 Control
TBI Level 2 Control
Tricontrols calibration verification set
TRICONTROLS LEVEL 1 CONTROL
TRICONTROLS LEVEL 2 CONTROL
TRICONTROLS LEVEL 3 CONTROL
Additional Resources
VALUE ASSIGNMENT SHEETS
Use Value Assignment Sheets (VAS) to locate the correct target values and ranges for your i-STAT System test cartridge controls and calibration verification materials. You may also access electronic Value Assignment Sheets (eVAS).
i-STAT Alinity content updated 13-January-2026
To find the correct VAS and ranges, you need to:
- Locate the cartridge type and lot number found on your cartridge box or pouch.
- Choose the corresponding lot and type from the menu below.
- Identify the corresponding cartridge type and lot prefix letter WITHIN the value assignment sheet.


CLEW D51 EXPIRES 15 JULY 2026
ACT LEVEL 1 CONTROL
ACT LEVEL 2 CONTROL
CHEM8+ CALIBRATION VERIFICATION LEVEL 1B
hs-TnI Calibration Verification Set
hs-TnI LEVEL 1 CONTROL
hs-TnI LEVEL 2 CONTROL
hs-TnI LEVEL 3 CONTROL
i-STAT calibration verification set
i-STAT LEVEL 1 CONTROL
i-STAT LEVEL 2 CONTROL
i-STAT LEVEL 3 CONTROL
TBI Calibration Verification Set
TBI Level 1 Control
TBI Level 2 Control
Tricontrols calibration verification set
TRICONTROLS LEVEL 1 CONTROL
TRICONTROLS LEVEL 2 CONTROL
TRICONTROLS LEVEL 3 CONTROL
Additional Resources
ELECTRONIC VALUE ASSIGNMENT SHEETS
You can automatically update your device(s) with the latest Value Assignment Sheet data to streamline and simplify the liquid quality control process.
i-STAT ALINITY DOWNLOAD INSTRUCTIONS
You must customize your instrument before using eVAS. To do so, use AlinIQ CWi to create a profile with eVAS selected and enabled for use, then load the profile onto the instrument. For further instructions, see the Manage and Assemble Profiles section of the System Operations Manual.
To download eVAS package file:
- Delete all pre-existing eVAS package files from your computer’s Downloads directory.
- Check the instrument CLEW.
- Power up the instrument.
- Touch More Options -> Instrument Status
- Observe CLEW indicated on the screen.
- Find the eVAS package file below that corresponds with the CLEW indicated on the Instrument Status screen.
- Download the eVAS package file.
- Click the eVAS Package File link corresponding to the instrument CLEW indicated on the screen. DO NOT RENAME THE FILE.
- Copy the eVAS package file to a USB memory stick.
- USB memory stick must be formatted as “FAT32” via Windows OS prior to use.
To install eVAS package file:
- Power up the instrument.
- From the Home screen:
Touch More Options -> Quality Options -> Update eVAS -> Install from USB
Follow prompts
i-STAT Alinity content updated 13-January-2026
eVAS File For Download
Additional Resources
ELECTRONIC VALUE ASSIGNMENT SHEETS
You can automatically update your device(s) with the latest Value Assignment Sheet data to streamline and simplify the liquid quality control process.
i-STAT ALINITY DOWNLOAD INSTRUCTIONS
You must customize your instrument before using eVAS. To do so, use AlinIQ CWi to create a profile with eVAS selected and enabled for use, then load the profile onto the instrument. For further instructions, see the Manage and Assemble Profiles section of the System Operations Manual.
To download eVAS package file:
- Delete all pre-existing eVAS package files from your computer’s Downloads directory.
- Check the instrument CLEW.
- Power up the instrument.
- Touch More Options -> Instrument Status
- Observe CLEW indicated on the screen.
- Find the eVAS package file below that corresponds with the CLEW indicated on the Instrument Status screen.
- Download the eVAS package file.
- Click the eVAS Package File link corresponding to the instrument CLEW indicated on the screen. DO NOT RENAME THE FILE.
- Copy the eVAS package file to a USB memory stick.
- USB memory stick must be formatted as “FAT32” via Windows OS prior to use.
To install eVAS package file:
- Power up the instrument.
- From the Home screen:
Touch More Options -> Quality Options -> Update eVAS -> Install from USB
Follow prompts
i-STAT Alinity content updated 13-January-2026

















