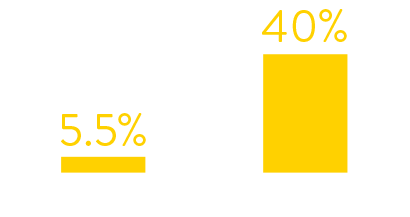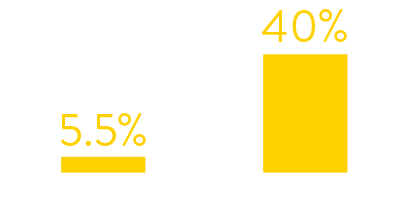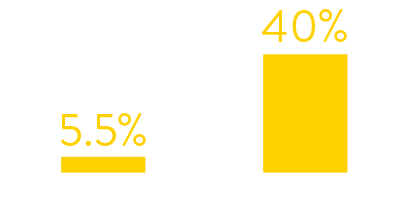Global Point of Care



Rapid point of care
Testing (POCT) for
virus management
Royal Derby Hospital improves infection control
with RSV testing
Royal Derby Hospital has been leading the way in infection control through point of care testing (POCT) since 2018. The hospital recognises the importance of POCT not just for patient safety, but also for staff in a medical environment.
In 2021, Royal Derby Hospital's paediatric team implemented POCT for the respiratory syncytial virus (RSV) in the emergency department. As a result of year-round RSV testing, the department discovered an unexpected surge in summer cases.
Yusuf Gray is Advanced Biomedical Scientist, Point of Care Technologies, University Hospitals Derby & Burton NHS Foundation Trust. He says: “The summer surge in RSV cases was surprising to see as RSV is predominately considered a winter illness, along with flu. Regular testing for these illnesses usually ends as we come out of the winter months.”
This increase in testing resulted in better care for patients, as Yusuf Gray explains, “by continuing to test people that came through the Paediatric or the Emergency Department we were more quickly able to diagnose those with RSV and get them access to antiviral medication if needed.”

Read the case study
CASE STUDY RESULTS
Data from hospital testing showed that in January 2022
just 5.5% of symptomatic patients tested positive for RSV.
By July 2022 the figures had increased to 40%.
symptomatic patients who tested positive for rsv
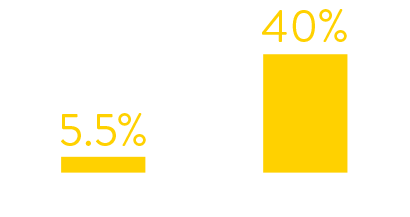
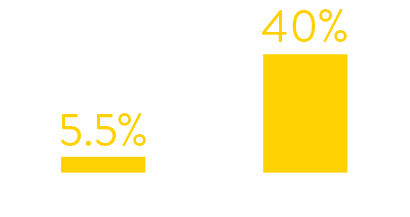
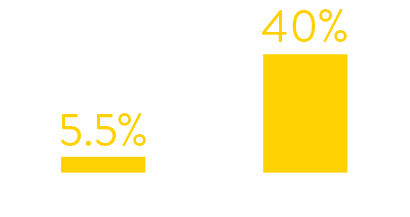


CASE STUDY RESULTS
Data from hospital testing showed that in January 2022
just 5.5% of symptomatic patients tested positive for RSV.
By July 2022 the figures had increased to 40%.
symptomatic patients who tested positive for rsv