Global Point of Care
SUPERARE LE SFIDE NELLA VALUTAZIONE DI UN SOSPETTO TRAUMA CRANICO LIEVE (MILD TRAUMATIC BRAIN INJURY, mTBI)
CON i-STAT TBI PLASMA TEST PER L'USO DELL'i-STAT ALINITY SYSTEM
L' i-STAT TBI Plasma test non è destinato all'uso come dispositivo POC
The i-STAT TBI Plasma test is not intended for use as a point-of-care-device



I METODI ATTUALI PER LA VALUTAZIONE DI UN SOSPETTO mTBI POSSONO ESSERE SOGGETTIVI
Milioni di pazienti vengono sottoposti ogni anno a una valutazione in Pronto Soccorso (PS) per mTBI. Ma il punteggio Glasgow Coma Scale (GCS) e la valutazione clinica forniscono solo informazioni soggettive, portando molti a utilizzare la TAC.
L'i-STAT TBI Plasma test può essere la soluzione alle TC della testa lunghe e spesso non necessarie per un mTBI.1
Gli Inconvenienti Di Una Tc
La Tc Per Tbi Ha Un Basso Rendimento Diagnostico E Presenta Inconvenienti Significativi


Le regole per la decisione clinica (Clinical decision rules, CDR), come la Canadian CT Head Rule, hanno avuto un impatto limitato sul numero o sul rendimento diagnostico della TC per la valutazione di un mTBI3-5
Si Stima Che L'82% Dei Pazienti Con Tbi Sia Sottoposto A Tc, Ma >90% Non Mostra Segni Di Anormalità Traumatica1
Una revisione sistematica di 14 studi ha rilevato che tra i pazienti con trauma cranico lieve, solo il 7% presentava gravi lesioni intracraniche identificate in una TC, mentre <1% presentava lesioni che richiedevano un intervento neurochirurgico2
COME STRUMENTO PER LA VALUTAZIONE DI UN SOSPETTO mTBI, LA TC PRESENTA INCONVENIENTI SIGNIFICATIVI
- Il tempo dalla prescrizione alla lettura della TC può arrivare fino a 3 ore, circa la metà del tempo totale per la valutazione di un mTBI6
- Le dosi di radiazioni agli organi derivanti dalla scansione TC sono sostanzialmente superiori rispetto a quelle della radiografia convenzionale7
- La dose di radiazioni della TC alla testa è equivalente a 100 volte quella di una radiografia del torace
- I pazienti difficili da valutare, come quelli in stato di ebbrezza, rappresentano un problema
BIOMARCATORI PER mTBI
I BIOMARCATORI DI LESIONI CEREBRALI HANNO IL POTENZIALE DI RIDURRE LA QUANTITÀ DI TC NON NECESSARIE DA ESEGUIRE PER UN SOSPETTO mTBI
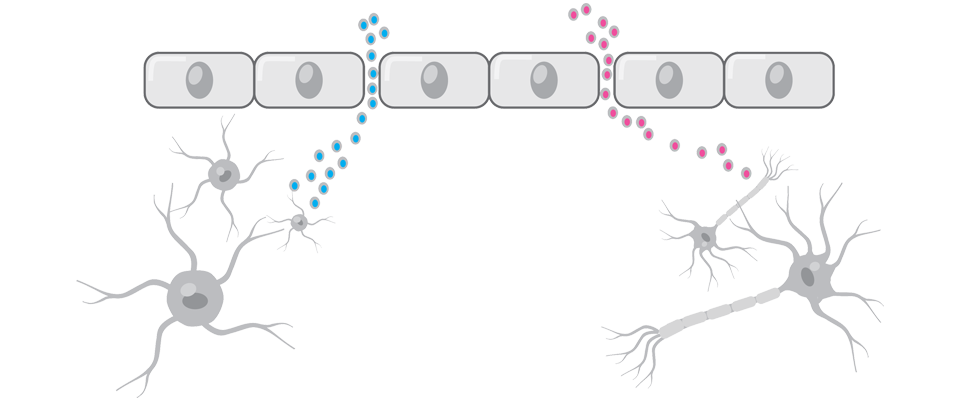
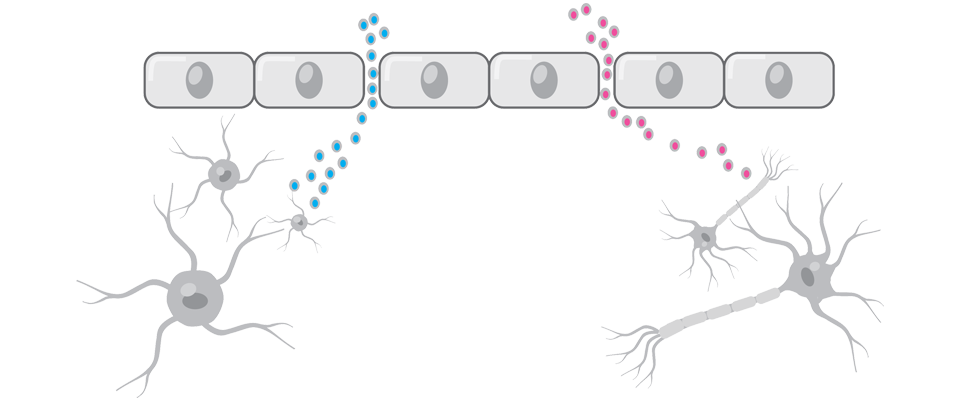

A Seguito Di Un Tbi, C'è Una Maggiore Permeabilità E Una Perdita Di Molecole Attraverso La Barriera Ematoencefalica8,9
Le proteine del cervello che attraversano la barriera ematoencefalica quando questa viene rotta possono fungere da biomarcatori per un TBI8
Anche Se Sono Stati Studiati Diversi Biomarcatori Di Tbi, Per Avere Valore Diagnostico In Pronto Soccorso Sono Considerate Essenziali Alcune Caratteristiche10:
- Facilmente misurabile in un fluido biologico accessibile, come il sangue periferico, subito dopo la lesione
- Elevati livelli ematici periferici specifici per le lesioni cerebrali
- Sensibilità abbastanza elevata per valutare lesioni lievi
L'i-STAT TBI PLASMA TEST
INTRODUZIONE ALL'i-STAT TBI PLASMA TEST, UN ESAME BASATO SU BIOMARCATORI PENSATO PER VALUTARE IN MODO OGGETTIVO LA NECESSITÀ DI UNA TC


L'i-STAT TBI Plasma test combina 2 esami per biomarcatori specifici del cervello, proteina fibrillare acida della glia (Glial Fibrillary Acidic Protein, GFAP) e ubiquitina carbossi-terminale idrolasi L1 (Ubiquitin Carboxyl-Terminal Hydrolase L1, UCH-L1), in un singolo test multiplex progettato per aiutare a determinare la reale necessità di una TC della testa.11
USO PREVISTO11
L'interpretazione dei risultati del test viene utilizzata, insieme ad altre informazioni cliniche, per aiutare nella valutazione dei pazienti, di età pari o maggiore di 18 anni, che presentano un sospetto mTBI (punteggio GCS 13-15) entro 12 ore dalla lesione, per contribuire a determinare la necessità di una TC della testa. *
*L' i-STAT TBI Plasma test deve essere utilizzato con plasma preparato da campioni anticoagulati di acido etilendiamminotetraacetico (EDTA) in ambienti clinici di laboratorio da un operatore sanitario. Non è destinata all'uso point-of-care.
i-STAT TBI PLASMA TEST RIDUCE L'INCERTEZZA FORNENDO UNA VALUTAZIONE QUANTIFICABILE E OGGETTIVA DA TENERE IN CONSIDERAZIONE PER UNA VALUTAZIONE DI SOSPETTO mTBI
- Con un valore predittivo negativo (Negative Predictive Value, NPV) del 99,3% nello studio pilota, l'i-STAT TBI Plasma test può ridurre la dipendenza dall'imaging TC, riducendo potenzialmente al minimo l'esposizione alle radiazioni non necessarie, la durata della degenza e l'utilizzo delle risorse per i pazienti che non sono a rischio di anormalità nella TC11
- L'elevata sensibilità clinica e l'NPV dell'i-STAT TBI Plasma test danno fiducia nel supportare decisioni per la dimissione sicura dei pazienti senza l'esecuzione di una TC

*L' i-STAT TBI Plasma test deve essere utilizzato con plasma preparato da campioni anticoagulati di EDTA in ambienti clinici di laboratorio da un operatore sanitario. Non è destinato all'uso point-of-care.
L'i-STAT TBI PLASMA TEST HA IL POTENZIALE DI RIDURRE FINO AL 40% IL NUMERO DI TC NON NECESSARIE PER UN SOSPETTO mTBI
Il 40,4% dei risultati negativi nello studio pivotale aveva un risultato vero negativo nell' i-STAT TBI Plasma test, suggerendo una potenziale riduzione delle TC non necessarie fino al 40%11,12



La Riduzione Della Quantità Di Tc Può Avere Implicazioni Positive Per Il Flusso Di Lavoro Del Pronto Soccorso, L'utilizzo Delle Risorse E La Soddisfazione Del Paziente
Per Ogni Scansione Evitata, Possono Essere Eliminati Anche I Tempi Di Attesa Associati Alla Stessa, L'utilizzo Delle Risorse E I Costi
Il Tempo Di 15 Minuti* Dello Strumento Dell'i-stat Tbi Plasma Test Può Fornire Importanti Vantaggi:
Tempi di attesa più brevi
Rapida rassicurazione
Dimissioni tempestive
QUALE IMPATTO POTREBBE AVERE SUL TUO PRONTO SOCCORSO UN ESAME DEI BIOMARCATORI CHE POSSA CONTRIBUIRE IN MODO OGGETTIVO E ACCURATO A ESCLUDERE LA NECESSITÀ DI UNA TC NELLA VALUTAZIONE DI UN mTBI?
I Biomarcatori Alla Base Del Test
Gfap E Uch-l1 Sono Biomarcatori Adeguatamente Convalidati, Complementari E Specifici Per Il Cervello, Rilasciati Nel Flusso Sanguigno Da 2 Diversi Tipi Di Cellule A Seguito Di Lesioni Cerebrali Traumatiche
Gfap È Una Proteina Strutturale Che Si Trova Quasi Esclusivamente Negli Astrociti13
- GFAP è un marcatore specifico di danno agli astrociti nella materia bianca o grigia che è elevato in pazienti con anomalie intracraniche traumatiche rilevate nella TC11,14
- GFAP distingue in modo affidabile tra pazienti traumatizzati con mTBI e quelli senza trauma cranico15
- A differenza di alcuni biomarcatori studiati in precedenza, i livelli di GFAP non sono influenzati da traumi extracranici o dall'esercizio fisico16-19
Uch-l1 È Un Enzima Di Degradazione Altamente Ed Esclusivamente Espresso Nei Neuroni13
- È stato dimostrato che i livelli ematici distinguono i pazienti con mTBI da quelli senza lesioni20
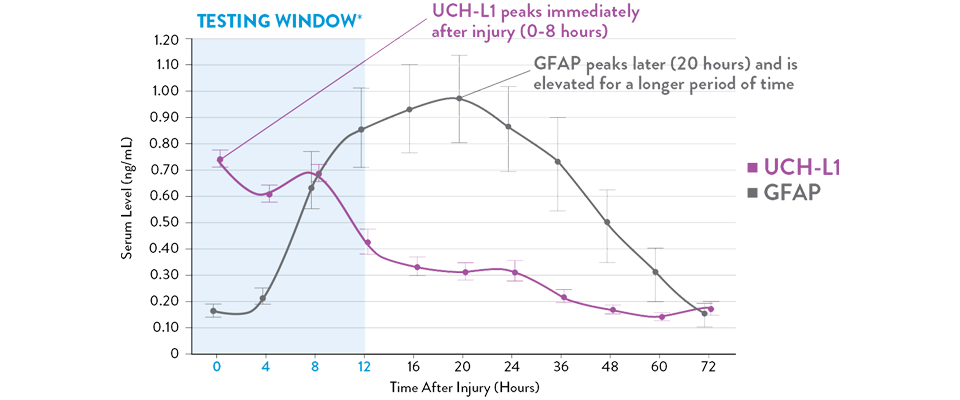
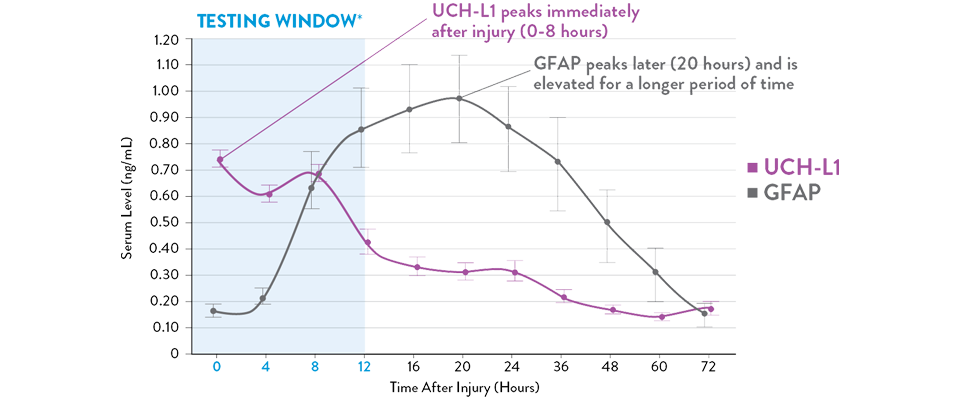

Adattato da Papa et al., 2016.
I livelli sierici di UCH-L1 raggiungono il picco da 0 a 8 ore successive alla lesione e diminuiscono costantemente nell'arco di 48 ore, mentre GFAP raggiunge il picco a 20 ore e diminuisce lentamente 72 ore dopo la lesione cerebrale.21 L'i-STAT TBI Plasma test misura i livelli di entrambi i biomarcatori durante il periodo ottimale di 12 ore successive alla lesione.11
*L'i-STAT TBI Plasma test deve essere utilizzato con plasma preparato da campioni anticoagulati di EDTA in ambienti clinici di laboratorio da un operatore sanitario. Non è destinato all'uso point-of-care.
References:
1. Korley FK, Kelen GD, Jones CM, Diaz-Arrastia R. Emergency department evaluation of traumatic brain injury in the United States, 2009-2010. J Head Trauma Rehabil. 2016;31(6):379-387.
2. Easter JS, Haukoos JS, Meehan WP, Novack V, Edlow JA. Will neuroimaging reveal a severe intracranial injury in this adult with minor head trauma? The Rational Clinical Examination Systematic Review. JAMA. 2015;314(24):2672-2681.
3. Sharp AL, Nagaraj G, Rippberger EJ, et al. Computed tomography use for adults with head injury: describing likely avoidable emergency department imaging based on the Canadian CT Head Rule. Acad Emerg Med. 2017;24(1):22-30.
4. Sultan HY, Boyle A, Pereira M, Antoun N, Maimaris C. Application of the Canadian CT head rules in managing minor head injuries in a UK emergency department: implications for the implementation of the NICE guidelines. Emerg Med J. 2014;21(4):420-425.
5. Stiel IG, Clement CM, Grimshaw JM, et al. A prospective cluster-randomized trial to implement the Canadian CT Head Rule in emergency departments. CMAJ. 2010;182(14):1527-1532.
6. Michelson EA, Huff JS, Loparo M, et al. Emergency department time course for mild traumatic brain injury workup. West J Emerg Med. 2010;19(4):635-640.
7. US Food and Drug Administration. What are the radiation risks from CT? Updated December 5, 2017. Accessed November 20, 2020. https://www.fda.gov/radiation-emitting-products/medical-x-ray-imaging/what-are-radiation-risks-ct.
8. Zetterberg H, Blennow K. Fluid biomarkers for mild traumatic brain injury and related conditions. Nat Rev Neurol. 2016;12(10):563-574.
9. Chodobski A, Zink BJ, Symydynger-Chodobska J. Blood-brain barrier pathophysiology in traumatic brain injury. Transl Stroke Res. 2011;2(4):492-516.
10. Wang KK, Yang Z, Zhu T, et al. An update on diagnostic and prognostic biomarkers for traumatic brain injury. Expert Rev Mol Diagn. 2018;18(2):165-180.
11. i-STAT TBI Plasma Cartridge. Instructions for use. Abbott Point of Care Inc. Abbott Park, IL; 2020.
12. Data on file. Abbott Point of Care Inc.
13. Diaz-Arrastia R, Wang KKW, Papa L, et al; TRACK-TBI Investigators. Acute biomarkers of traumatic brain injury: relationship between plasma levels of ubiquitin C-terminal hydrolase-L1 and glial fibrillary acidic protein. J Neurotrauma. 2014;31(1):19-25.
14. Metting Z, Wilczak N, Rodiger LA, Schaaf JM, van der Naalt J. GFAP and S100B in the acute phase of mild traumatic brain injury. Neurology. 2012;78(18):1428-1433.
15. Papa L, Lewis LM, Silvestri S, Falk JL, Giordano P, Brophy GM. Serum levels of Ubiquitin C-terminal hydrolase (UCH-L1) distinguish mild traumatic brain injury (TBI) from trauma controls and are elevated in mild and moderate TBI patients with intracranial lesions and neurosurgical intervention. J Trauma Acute Care Surg. 2012;72(5):1335-1344.
16. Jones A, Jarvis P. Review of the potential use of blood neuro-biomarkers in the diagnosis of mild traumatic brain injury. Clin Exp Emerg Med. 2017;4(3):121-127.
17. Schulte S, Podlog LW, Hamson-Utley JJ, Strathmann FG, Strϋder HK. A systematic review of the biomarker S100B: implications for sport-related concussion management. J Athl Train. 2014;49(6):830-850.
18. Steiner J, Bernstein H-G, Bielau H, et al. Evidence for a wide extra-astrocytic distribution of S100B in human brain. BMC Neurosci. 2007;8:2.
19. Pelinka LE, Kroepfl A, Schmidhammer R, et al. Glial fibrillary acidic protein in serum after traumatic brain injury and multiple trauma. J Trauma. 2004;57(5):1006-1012.
20. Papa L, Lewis LM, Falk JL, et al. Elevated levels of serum glial fibrillary acidic protein breakdown products in mild and moderate traumatic brain injury are associated with intracranial lesions and neurosurgical intervention. Ann Emerg Med. 2012;59(6):471-483.
21. Papa L, Brophy GM, Welch RD, et al. Time course and diagnostic accuracy of glial and neuronal blood biomarkers GFAP and UCH-L1 in a large cohort of trauma patients with and without mild traumatic brain injury. JAMA Neurol. 2016;73(5):551-560.


