GLOBAL POINT OF CARE
NAVIGATE CHALLENGING CASES
with i‑STAT tbi plasma, an objective biomarker test for mild traumatic brain injury (mTBI)

NAVIGATE CHALLENGING CASES
with i‑STAT tbi plasma, an objective biomarker test for mild traumatic brain injury (mTBI)

NAVIGATE CHALLENGING CASES
with i‑STAT tbi plasma, an objective biomarker test for mild traumatic brain injury (mTBI)



SUBJECTIVE METHODS FOR EVALUATING SUSPECTED mTBI CAN LEAVE YOU IN A QUANDARY
Millions of patients undergo emergency department (ED) evaluation for mTBI each year. Neurocognitive assessments, such as the Glasgow Coma Scale (GCS) and clinical decision rules (CDRs), are subjective and can be difficult to perform with certain patients, such as those who are intoxicated or have an altered mental health status.¹
As a result, you may decide to order a CT, despite having low clinical suspicion of intracranial bleeding. In fact, while an estimated 82% of patients with TBI undergo CT, more than 90% show no evidence of traumatic abnormality.² What’s more, some patients may insist on a CT even though you deem it unwarranted.
The i-STAT TBI Plasma test may be the solution to provide objective data without time-consuming and often avoidable head CTs for mTBI.
CHALLENGING PATIENTS
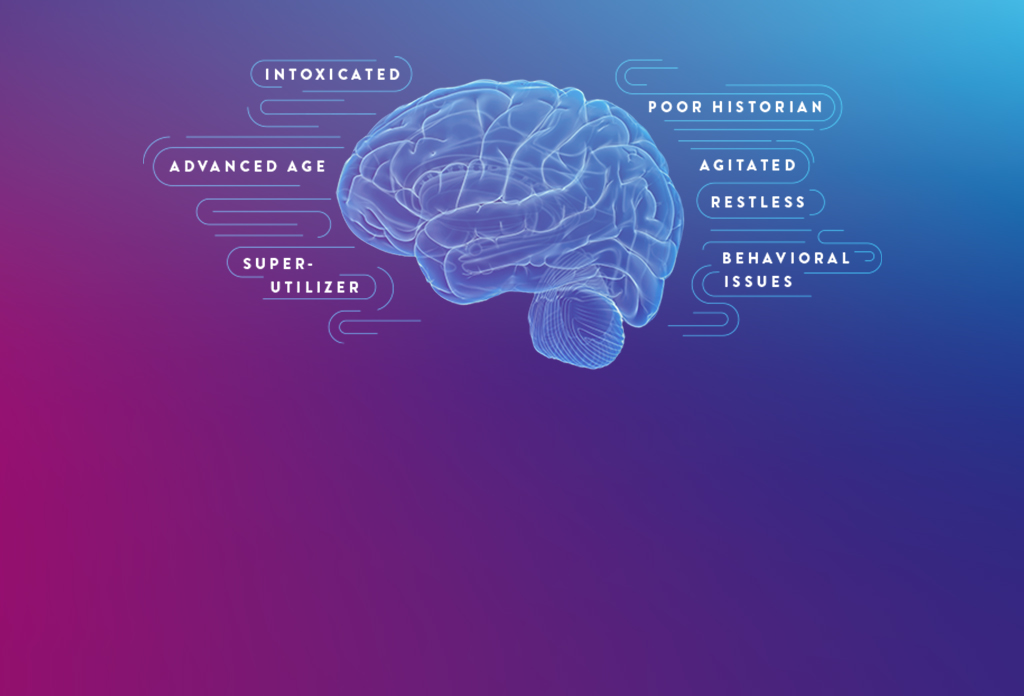
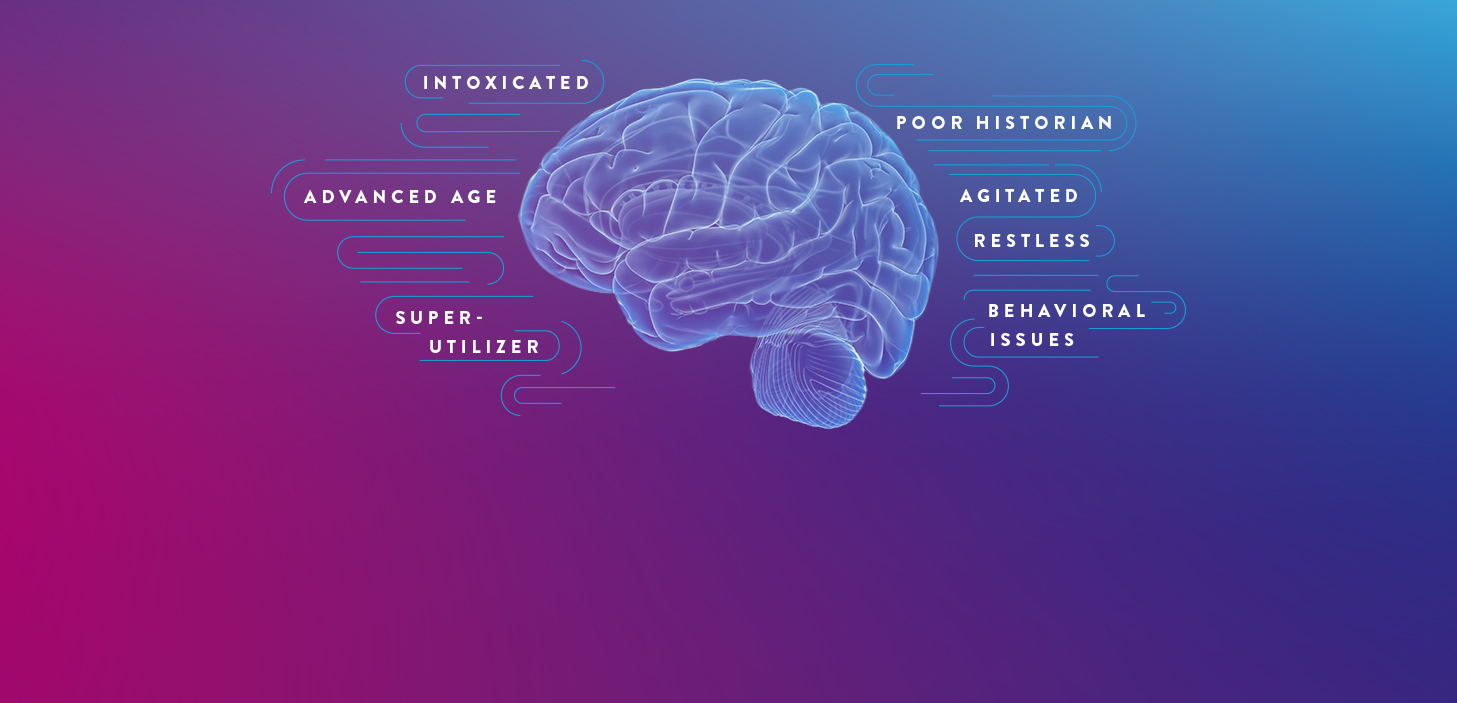
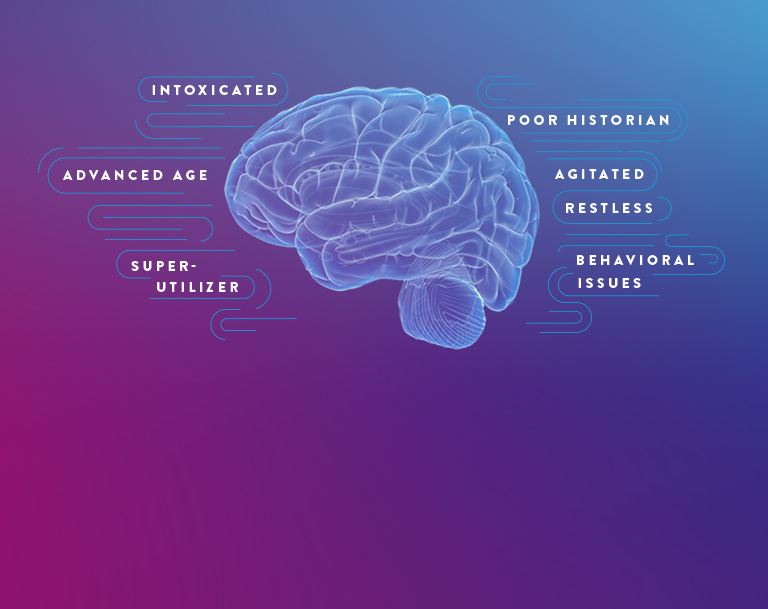
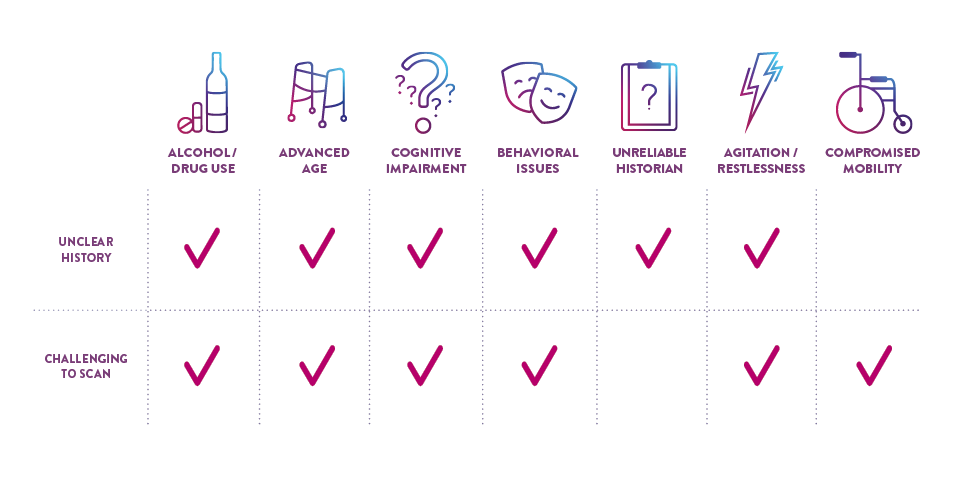
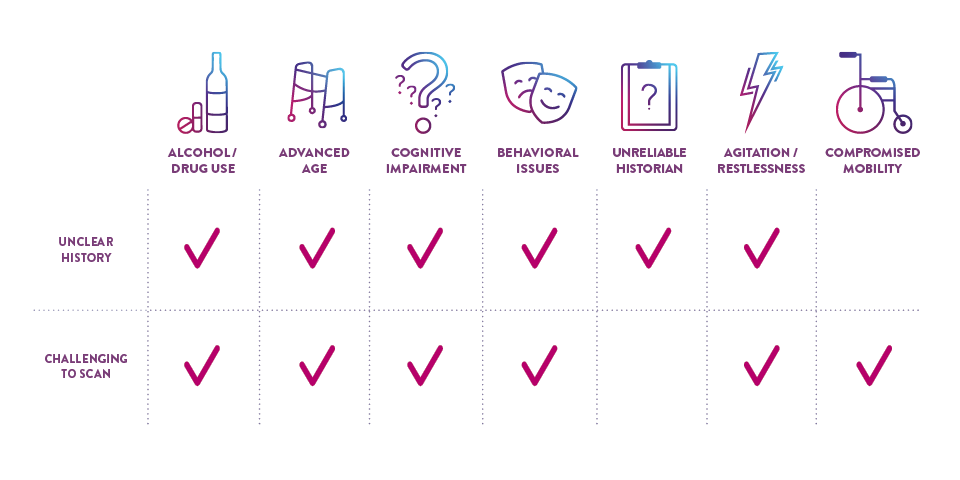
CERTAIN PATIENT POPULATIONS ARE CHALLENGING TO ASSESS FOR mTBI
In addition to the limitations of clinical decision rules (CDRs) in evaluating some patients with suspected mTBI, patients may present in a way that complicates the logistics of performing a CT scan and may even require sedation, which can further complicate and delay evaluation.

CT CONSIDERATIONS
CT MAY EXPOSE PATIENTS TO UNNECESSARY RADIATION
HEAD CT=100 X
THE RADIATION EXPOSURE OF A CHEST X-RAY 3
RISK OF CUMULATIVE RADIATION EXPOSURE
IN PATIENTS WITH FREQUENT INJURY LIKE ATHLETES AND SUPER-UTILIZERS
ACCESS TO CT MAY NOT ALWAYS BE IMMEDIATE
high patient volume in busy EDs can delay care of non-critical patients
- Patients presenting with mild symptoms are often deprioritized in favor of more severe or acute cases
- Extends patient wait times and delays treatment
- Radiologists may not be available to read CT scans, adding to a backlog
SOME FACILITIES HAVE NO CT ON THE PREMISES
- On-site assessment is limited to observation and CDRs
- Potential for added transportation costs and resources
- Disruption and delay to patient care
Biomarkers for mTBI
BRAIN-SPECIFIC BIOMARKERS CAN AID IN THE EVALUATION OF mTBI, EVEN WITH CHALLENGING CASES 4-8
GFAP and UCH-L1 are well validated, complementary, brain-specific biomarkers released into the bloodstream from two different cell types following traumatic brain injury.
GLIAL FIBRILLARY ACIDIC PROTEIN (GFAP)
- GFAP is a specific marker of astrocyte injury in either white or gray matter that is elevated in patients with traumatic intracranial abnormalities on CT6,7
- GFAP reliably distinguishes between trauma patients with mTBI and those without head injury8
- Unlike some previously studied biomarkers, GFAP levels are not affected by extracranial trauma or exercise9-12

UBIQUITIN CARBOXYL-TERMINAL HYDROLASE L1 (UCH-L1)
- UCH-L1 is a degradation enzyme highly and exclusively expressed in neurons13
- Blood levels have been demonstrated to distinguish mTBI patients from those without injuries14
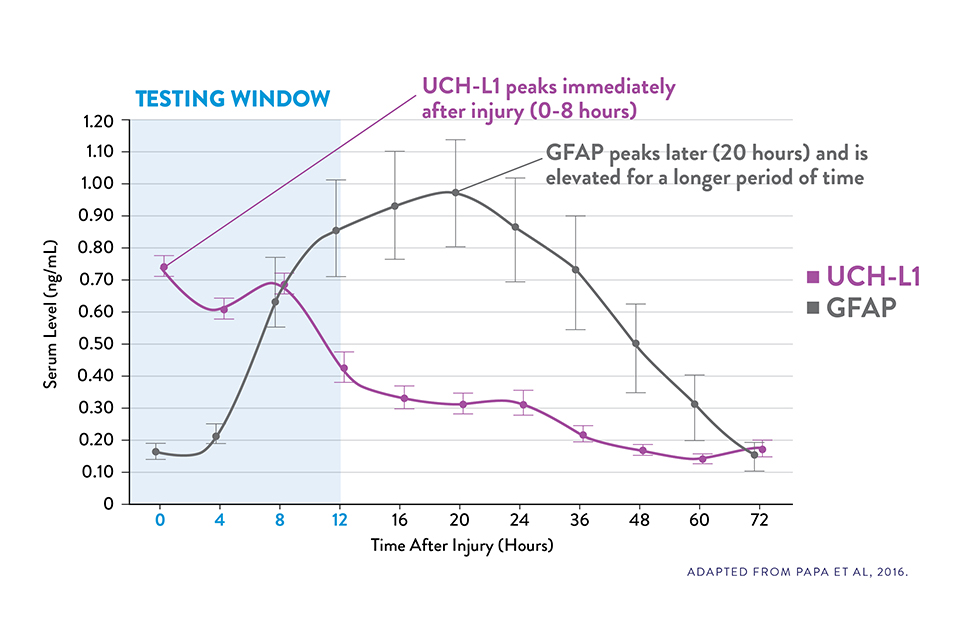
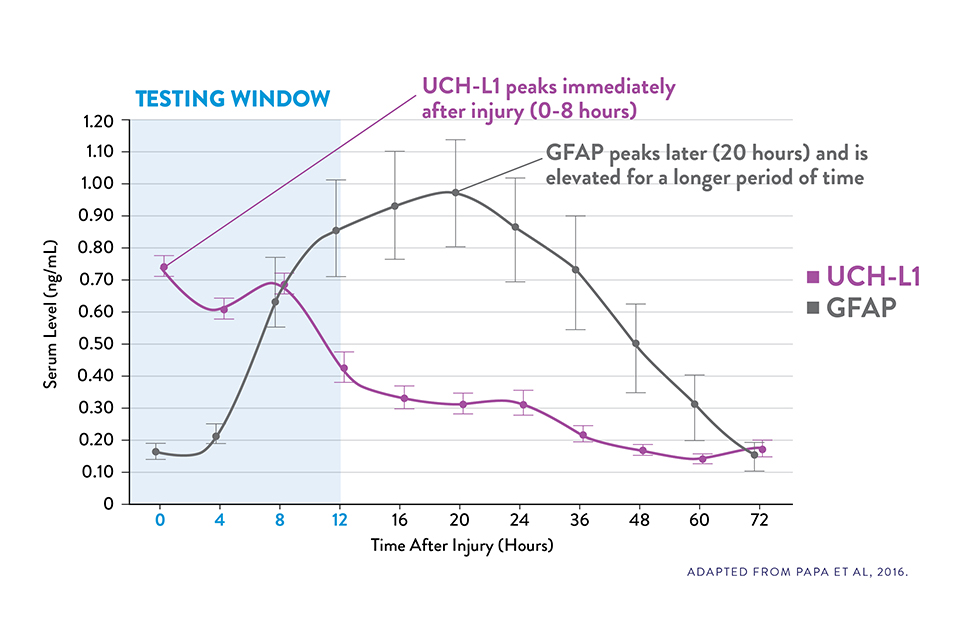
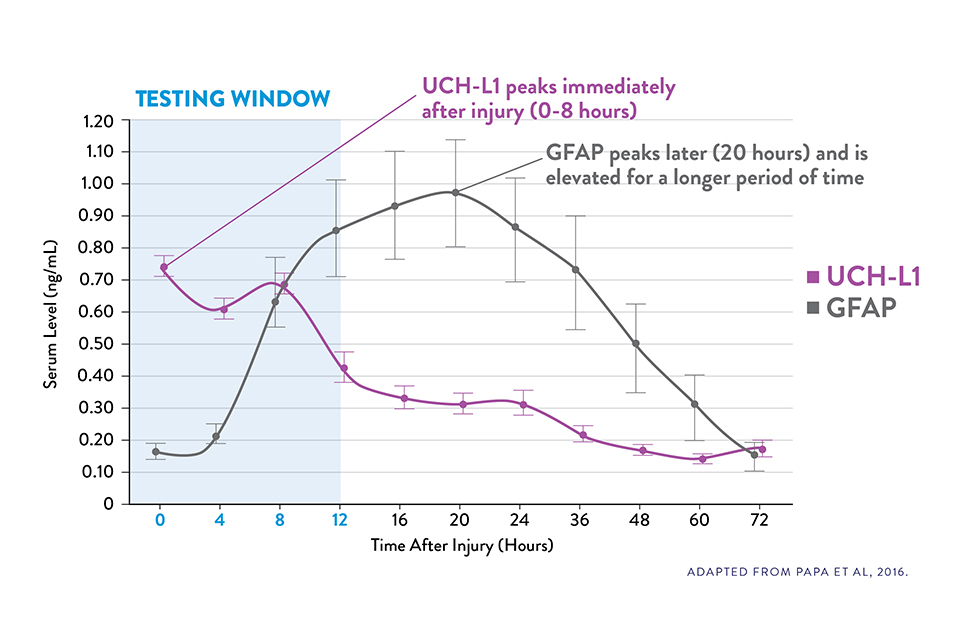
COMPLEMENTARY KINETICS OF GFAP AND UCH-L1 PROVIDE A RELIABLE 12-HOUR TESTING WINDOW15


40.4% of negative results in the pivotal study had a true negative result† on the i-STAT TBI Plasma test—suggesting a potential reduction in unnecessary CT of up to 40%6,16
Serum UCH-L1 levels peak 0 to 8 hours post injury and steadily decrease over 48 hours while GFAP peaks at 20 hours and declines slowly 72 hours after brain injury.15
The i-STAT TBI Plasma test measures levels of both biomarkers during the optimal 12-hour period following injury.6‡
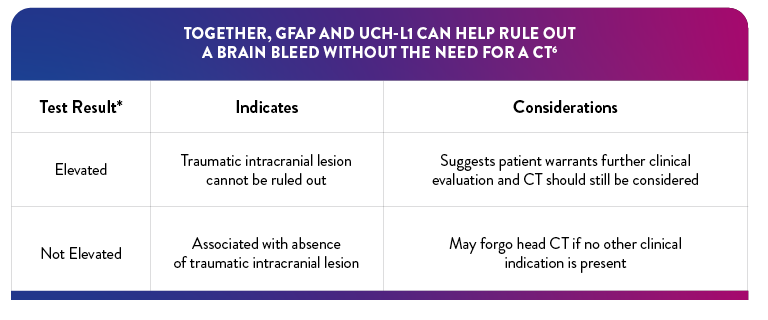
*If either biomarker is above the specified threshold, the result is "elevated"
†True negative result corresponds to clinical specificity
‡The i-STAT TBI Plasma test is to be used with plasma prepared from EDTA-anticoagulated specimens in clinical laboratory settings by a healthcare professional. It is not intended for point-of-care use.
THE i-STAT TBI Plasma Test
reduce avoidable ct use for suspected mTBI by up to 40%6,16
The i-STAT TBI Plasma test combines two brain-specific and complementary biomarkers, GFAP and UCH-L1, in a single, multiplex test designed to optimize sensitivity and negative predictive value (NPV) to help determine the need for CT.
The i-STAT TBI Plasma test is not intended for use as a point-of-care device. For in vitro diagnostic use only.





The high clinical sensitivity and NPV of the i-STAT TBI Plasma test provide confidence in aiding decisions for the safe discharge of patients without performing CT
15-minute instrument time6* may provide shorter wait times and timely discharge
*After obtaining plasma sample.
This test is to be used with plasma in clinical laboratory settings by a healthcare professional.
AN INNOVATIVE DUAL BIOMARKER ASSAY THAT IS REDEFINING THE EVALUATION OF mTBI

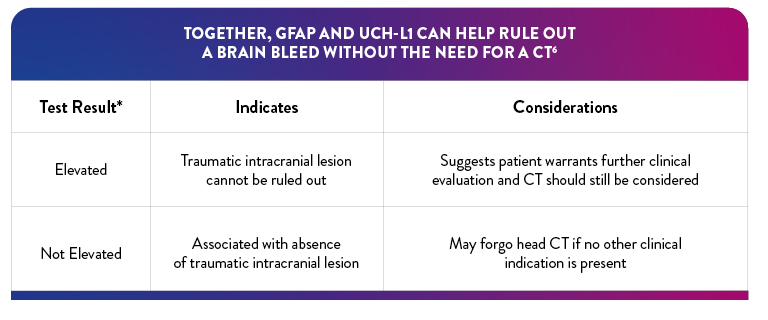
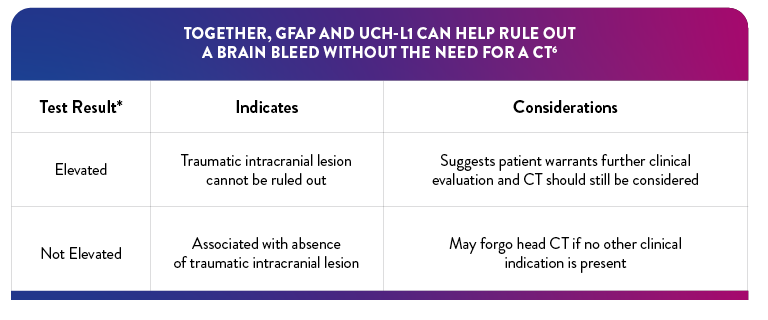
"Elevated" Test interpretation suggests further evaluation by CT should be considered6
"Not Elevated" Test interpretation is associated with the absence of acute traumatic intracranial lesions on CT6
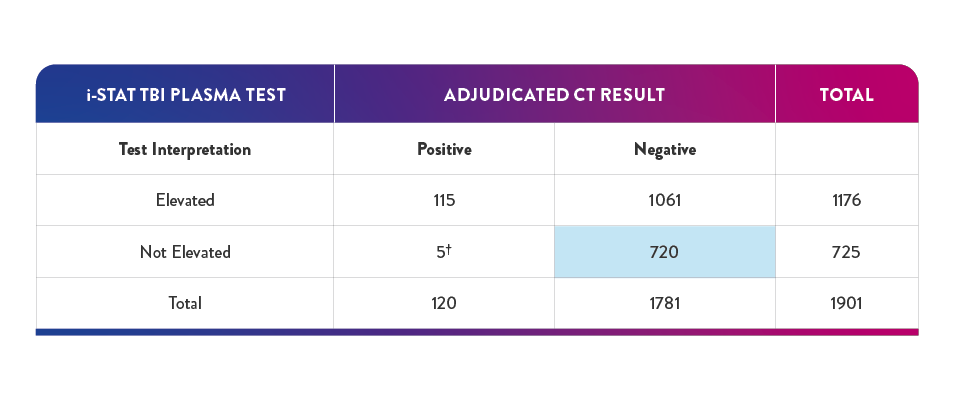
40.4% of negative results in the pivotal study had a true negative result on the i-STAT TBI Plasma test -suggesting a potential reduction in unnecessary CT of up to 40%6,16
†In the study, 5 participants with "not elevated" test findings and a "positive" CT finding, no nuerosurgical intervention was required
‡True negative result corresponds to clinical specificity
i‑STAT TBI PLASMA: A RELIABLE DUAL BIOMARKER ASSAY TO ASSIST YOU IN EVALUATING mTBI CASES
OBJECTIVE DATA
Offers a new adjunct assessment tool in mTBI evaluation, especially when there is clinical uncertainty
efficiency
Potential to improve ED workflow and optimize resource utilization by reducing avoidable CT and improving patient throughput


References:
1. Centers for Disease Control and Prevention. Get the facts about TBI. May 12, 2021. Accessed January 18, 2023. https://www.cdc.gov/traumaticbraininjury/get_the_facts.htm
2. Korley FK, Kelen GD, Jones CM, Diaz-Arrastia R. Emergency department evaluation of traumatic brain injury in the United States, 2009-2010. J Head Trauma Rehabil. 2016;31(6):379-387.
3. US Food and Drug Administration. What are the radiation risks from CT? Updated December 5, 2017. Accessed February 9, 2023.
https://www.fda.gov/radiation-emitting-products/medical-x-ray-imaging/what-are-radiation-risks-ct.
4. Zetterberg H, Blennow K. Fluid biomarkers for mild traumatic brain injury and related conditions. Nat Rev Neurol. 2016;12(10):563-574.
5. Chodobski A, Zink BJ, Symydynger-Chodobska J. Blood-brain barrier pathophysiology in traumatic brain injury. Trans Stroke Res. 2011;2(4):492-516.
6. i-STAT TBI Plasma Cartridge. Instructions for use. Abbott Point of Care Inc. Abbott Park, IL; 2021.
7. Metting Z, Wilezak N, Rodiger LA, et al. GFAP and S100B in the acute phase of mild traumatic brain injury. Neurology. 2012;78(18):1428-1433.
8. Papa L, Lewis LM, Falk JL, et al. Elevated levels of serum glial fibrillary acidic protein breakdown products in mild and moderate traumatic brain injury are associated with intracranial lesions and neurosurgical intervention. Ann Emerg Med. 2012;59(6):471-483.
9. Jones A, Jarvis P. Review of the potential use of blood neuro-biomarkers in the diagnosis of mild traumatic brain injury. Clin Exp Emerg Med. 2017;4(3):121-127.
10. Schulte S, Podlog LW, Hamson-Utley JJ, et al. A systematic review of the biomarker S100B: implications for sport-related concussion management. J Athl Train. 2014;49(6):830-850.
11. Steiner J, Bernstein H-G, Bielau H, et al. Evidence for a wide extra-astrocytic distribution of S100B in human brain. BMC Neurosci. 2007;8:2.
12. Pelinka LE, Kroepfl A, Schmidhammer R, et al. Glial fibrillary acidic protein in serum after traumatic brain injury and multiple trauma. J Trauma. 2004;57(5):1006-1012.
13. Diaz-Arrastia R, Wang KKW, Papa L, et al. Acute biomarkers of traumatic brain injury: relationship between plasma levels of ubiquitin C-terminal hydrolase-Li and glial fibrillary acidic protein. J Neurotrauma. 2014;31(1):19-25.
14. Papa L, Lewis LM, Silvestri S, et al. Serum levels of ubiquitin C-terminal hydrolase (UCH-L1) distinguish mild traumatic brain injury (TBI) from trauma controls and are elevated in mild and moderate TBI patients with intracranial lesions and neurosurgical intervention. J Trauma Acute Care Surg. 2012;72(5):1335-1344.
15. Papa L, Brophy GM, Welch RD, et al. Time course and diagnostic accuracy of glial and neuronal blood biomarkers GFAP and UCH-L1 in a large cohort of trauma patients with and without mild traumatic brain injury. JAMA Neurol. 2016;73(5):551-560.
16. Data on file. Abbott Point of Care Inc.
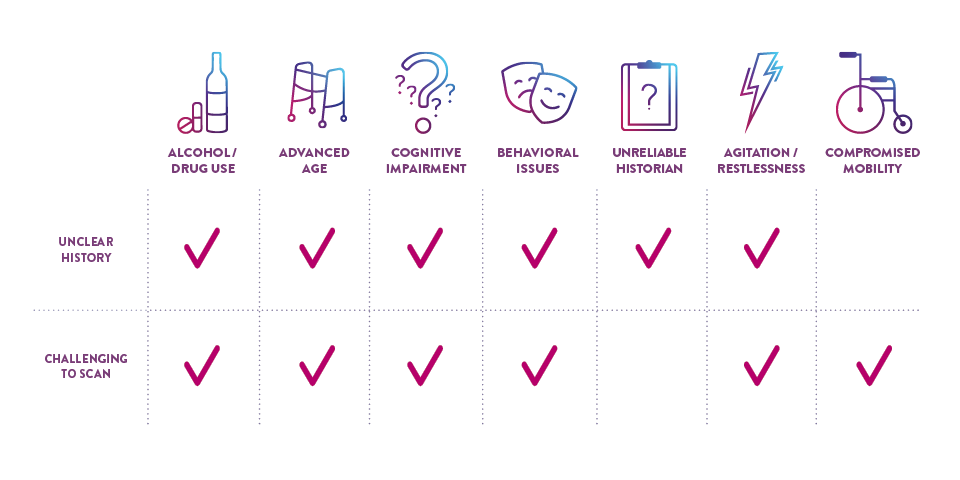
CHALLENGING PATIENTS
CERTAIN PATIENT POPULATIONS ARE CHALLENGING TO ASSESS FOR mTBI
In addition to the limitations of clinical decision rules (CDRs) in evaluating some patients with suspected mTBI, patients may present in a way that complicates the logistics of performing a CT scan and may even require sedation, which can further complicate and delay evaluation.


CT CONSIDERATIONS
CT MAY EXPOSE PATIENTS TO UNNECESSARY RADIATION
HEAD CT=100 X
THE RADIATION EXPOSURE OF A CHEST X-RAY 3
RISK OF CUMULATIVE RADIATION EXPOSURE
IN PATIENTS WITH FREQUENT INJURY LIKE ATHLETES AND SUPER-UTILIZERS
ACCESS TO CT MAY NOT ALWAYS BE IMMEDIATE
high patient volume in busy EDs can delay care of non-critical patients
- Patients presenting with mild symptoms are often deprioritized in favor of more severe or acute cases
- Extends patient wait times and delays treatment
- Radiologists may not be available to read CT scans, adding to a backlog
SOME FACILITIES HAVE NO CT ON THE PREMISES
- On-site assessment is limited to observation and CDRs
- Potential for added transportation costs and resources
- Disruption and delay to patient care
Biomarkers for mTBI
BRAIN-SPECIFIC BIOMARKERS CAN AID IN THE EVALUATION OF mTBI, EVEN WITH CHALLENGING CASES 4-8
GFAP and UCH-L1 are well validated, complementary, brain-specific biomarkers released into the bloodstream from two different cell types following traumatic brain injury.
GLIAL FIBRILLARY ACIDIC PROTEIN (GFAP)
- GFAP is a specific marker of astrocyte injury in either white or gray matter that is elevated in patients with traumatic intracranial abnormalities on CT6,7
- GFAP reliably distinguishes between trauma patients with mTBI and those without head injury8
- Unlike some previously studied biomarkers, GFAP levels are not affected by extracranial trauma or exercise9-12

UBIQUITIN CARBOXYL-TERMINAL HYDROLASE L1 (UCH-L1)
- UCH-L1 is a degradation enzyme highly and exclusively expressed in neurons13
- Blood levels have been demonstrated to distinguish mTBI patients from those without injuries14
COMPLEMENTARY KINETICS OF GFAP AND UCH-L1 PROVIDE A RELIABLE 12-HOUR TESTING WINDOW15




40.4% of negative results in the pivotal study had a true negative result† on the i-STAT TBI Plasma test—suggesting a potential reduction in unnecessary CT of up to 40%6,16
Serum UCH-L1 levels peak 0 to 8 hours post injury and steadily decrease over 48 hours while GFAP peaks at 20 hours and declines slowly 72 hours after brain injury.15
The i-STAT TBI Plasma test measures levels of both biomarkers during the optimal 12-hour period following injury.6‡
*If either biomarker is above the specified threshold, the result is "elevated"
†True negative result corresponds to clinical specificity
‡The i-STAT TBI Plasma test is to be used with plasma prepared from EDTA-anticoagulated specimens in clinical laboratory settings by a healthcare professional. It is not intended for point-of-care use.
THE i-STAT TBI Plasma Test
reduce avoidable ct use for suspected mTBI by up to 40%6,16
The i-STAT TBI Plasma test combines two brain-specific and complementary biomarkers, GFAP and UCH-L1, in a single, multiplex test designed to optimize sensitivity and negative predictive value (NPV) to help determine the need for CT.
The i-STAT TBI Plasma test is not intended for use as a point-of-care device. For in vitro diagnostic use only.





The high clinical sensitivity and NPV of the i-STAT TBI Plasma test provide confidence in aiding decisions for the safe discharge of patients without performing CT
15-minute instrument time6* may provide shorter wait times and timely discharge
*After obtaining plasma sample.
This test is to be used with plasma in clinical laboratory settings by a healthcare professional.
AN INNOVATIVE DUAL BIOMARKER ASSAY THAT IS REDEFINING THE EVALUATION OF mTBI



"Elevated" Test interpretation suggests further evaluation by CT should be considered6
"Not Elevated" Test interpretation is associated with the absence of acute traumatic intracranial lesions on CT6
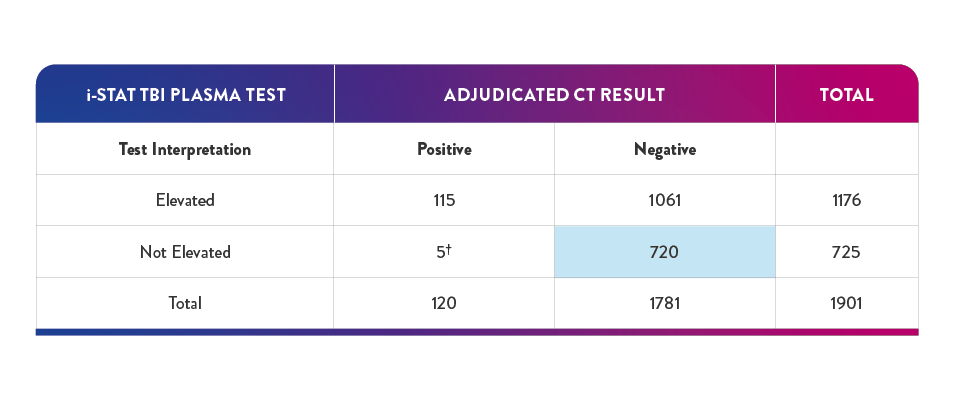
40.4% of negative results in the pivotal study had a true negative result on the i-STAT TBI Plasma test -suggesting a potential reduction in unnecessary CT of up to 40%6,16
†In the study, 5 participants with "not elevated" test findings and a "positive" CT finding, no nuerosurgical intervention was required
‡True negative result corresponds to clinical specificity
i‑STAT TBI PLASMA: A RELIABLE DUAL BIOMARKER ASSAY TO ASSIST YOU IN EVALUATING mTBI CASES
OBJECTIVE DATA
Offers a new adjunct assessment tool in mTBI evaluation, especially when there is clinical uncertainty
efficiency
Potential to improve ED workflow and optimize resource utilization by reducing avoidable CT and improving patient throughput


References:
1. Centers for Disease Control and Prevention. Get the facts about TBI. May 12, 2021. Accessed January 18, 2023. https://www.cdc.gov/traumaticbraininjury/get_the_facts.htm
2. Korley FK, Kelen GD, Jones CM, Diaz-Arrastia R. Emergency department evaluation of traumatic brain injury in the United States, 2009-2010. J Head Trauma Rehabil. 2016;31(6):379-387.
3. US Food and Drug Administration. What are the radiation risks from CT? Updated December 5, 2017. Accessed February 9, 2023.
https://www.fda.gov/radiation-emitting-products/medical-x-ray-imaging/what-are-radiation-risks-ct.
4. Zetterberg H, Blennow K. Fluid biomarkers for mild traumatic brain injury and related conditions. Nat Rev Neurol. 2016;12(10):563-574.
5. Chodobski A, Zink BJ, Symydynger-Chodobska J. Blood-brain barrier pathophysiology in traumatic brain injury. Trans Stroke Res. 2011;2(4):492-516.
6. i-STAT TBI Plasma Cartridge. Instructions for use. Abbott Point of Care Inc. Abbott Park, IL; 2021.
7. Metting Z, Wilezak N, Rodiger LA, et al. GFAP and S100B in the acute phase of mild traumatic brain injury. Neurology. 2012;78(18):1428-1433.
8. Papa L, Lewis LM, Falk JL, et al. Elevated levels of serum glial fibrillary acidic protein breakdown products in mild and moderate traumatic brain injury are associated with intracranial lesions and neurosurgical intervention. Ann Emerg Med. 2012;59(6):471-483.
9. Jones A, Jarvis P. Review of the potential use of blood neuro-biomarkers in the diagnosis of mild traumatic brain injury. Clin Exp Emerg Med. 2017;4(3):121-127.
10. Schulte S, Podlog LW, Hamson-Utley JJ, et al. A systematic review of the biomarker S100B: implications for sport-related concussion management. J Athl Train. 2014;49(6):830-850.
11. Steiner J, Bernstein H-G, Bielau H, et al. Evidence for a wide extra-astrocytic distribution of S100B in human brain. BMC Neurosci. 2007;8:2.
12. Pelinka LE, Kroepfl A, Schmidhammer R, et al. Glial fibrillary acidic protein in serum after traumatic brain injury and multiple trauma. J Trauma. 2004;57(5):1006-1012.
13. Diaz-Arrastia R, Wang KKW, Papa L, et al. Acute biomarkers of traumatic brain injury: relationship between plasma levels of ubiquitin C-terminal hydrolase-Li and glial fibrillary acidic protein. J Neurotrauma. 2014;31(1):19-25.
14. Papa L, Lewis LM, Silvestri S, et al. Serum levels of ubiquitin C-terminal hydrolase (UCH-L1) distinguish mild traumatic brain injury (TBI) from trauma controls and are elevated in mild and moderate TBI patients with intracranial lesions and neurosurgical intervention. J Trauma Acute Care Surg. 2012;72(5):1335-1344.
15. Papa L, Brophy GM, Welch RD, et al. Time course and diagnostic accuracy of glial and neuronal blood biomarkers GFAP and UCH-L1 in a large cohort of trauma patients with and without mild traumatic brain injury. JAMA Neurol. 2016;73(5):551-560.
16. Data on file. Abbott Point of Care Inc.

