Global Point Of Care
SUSPICIOUS SYMPTOMS? GET CLARITY FROM RESPIRATORY CONFUSION
WITH ID NOW™ RAPID MOLECULAR TESTS



DECODE YOUR SYMPTOMS
Suffering from suspicious symptoms this respiratory season? That hacking, distracting, could be anything lousy feeling. Clear up the confusion of what it is - the flu, COVID-19 or strep throat, by visiting a test provider with ID NOW™ Rapid Molecular Testing.
Accurate Results in minutes
If you aren't feeling well, a test provider can determine whether you need a test for Strep A, the Flu, COVID-19 or RSV. You’ll get a fast, accurate result in 13 minutes or less!
Get treatment sooner
ID NOW's fast, accurate results help clinicians make treatment decisions sooner. They will determine if you need antivirals or antibiotics so you can feel better faster.
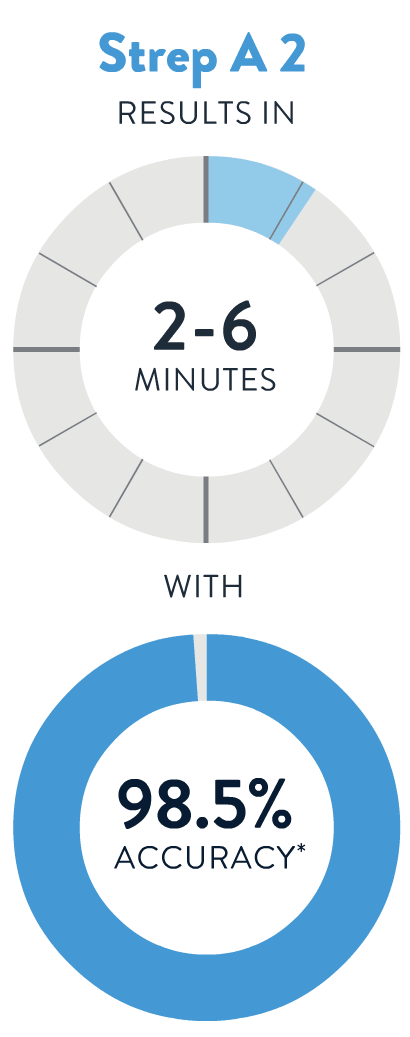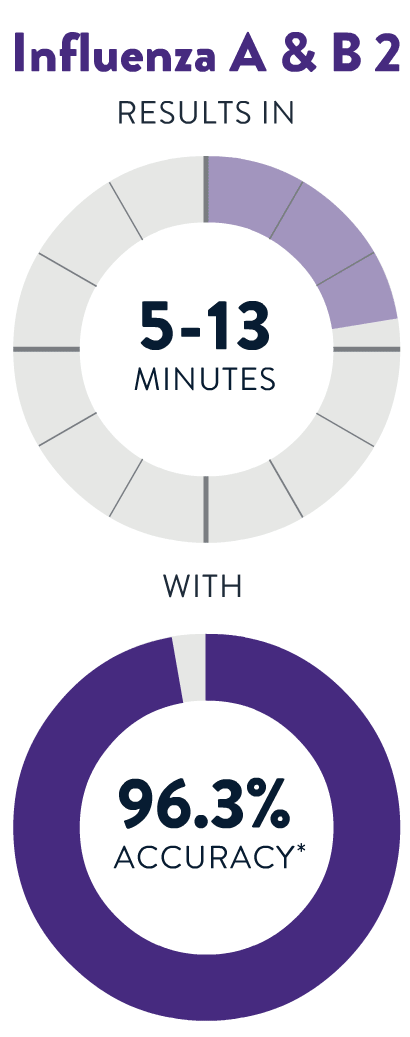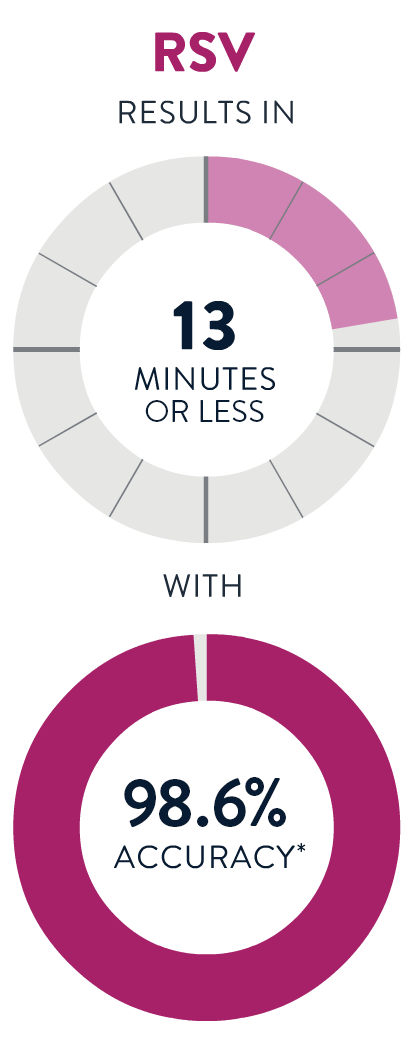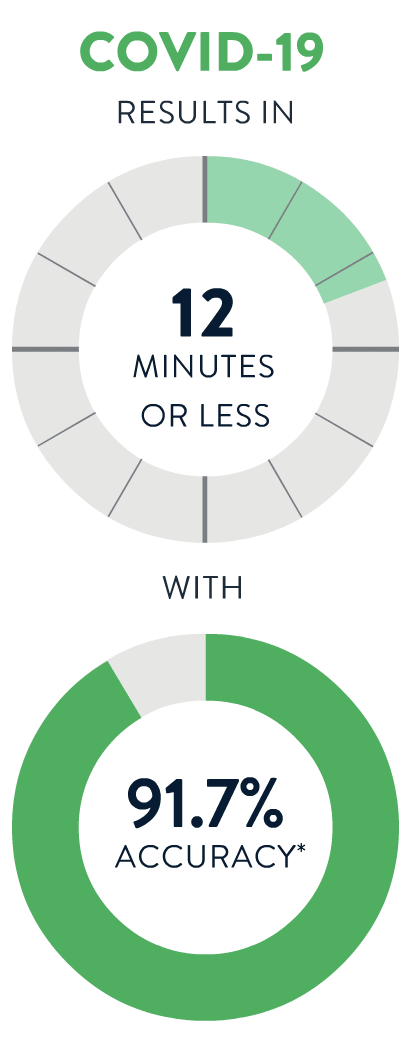
*Accuracy is derived from Sensitivity (per PI) or PPA
Find a test provider
Let a test provider help decode your symptoms with the clarity offered by an ID NOW™ Rapid Molecular Test. To get started, find a test provider who will determine if you need a test for strep throat, the flu, or COVID-19. Not seeing any test providers in your area? Check with your local pharmacy or clinician.
If you are NOT seeing the “Find a test provider nearby” map in this window, scroll to the bottom of this page and click on Your Privacy Choices in bottom left of the window. Select “Accept Sale/Sharing and Targeted Advertising”. The map will load after refreshing page.
HEALTHCARE PROFESSIONALS
To be added to our ID NOW Healthcare Professional locator tool, please provide the required information for each location you would like to be included on the locator tool.
PUT CLARITY IN
YOUR CORNER
Want to talk to a test provider about rapid molecular testing? Take the first step to get the information you need.




ID NOW TECHNOLOGY IN ACTION
SAMPLE COLLECTED
A sample is taken by swabbing the inside of your nose or throat.
TEST CONDUCTED
ID NOW™ checks the sample, looking for the DNA or RNA of the virus or bacteria.
RESULTS IN MINUTES
In 13 minutes or less, your clinician can receive a test result.
GET THE INSIGHTS AND ANSWERS YOU NEED
Did you know many respiratory illnesses share common symptoms? Find out why testing matters and how it can help you get well sooner.




RESULTS ON YOUR SCHEDULE. ANSWERS IN YOUR HANDS.
Which test is best? Learn the differences and how to get fast, simple, and more accessible testing that is right for you.
IS IT JUST A COLD, OR SOMETHING MORE?
Get monthly supportive tips and timely information directly to your inbox with Rapid Insights and stay a step ahead when you feel sick.
REFERENCES
- ID NOW™ Strep A 2 clinical trial data, held on file
- ID NOW™ Strep A 2 Product Insert
- Iglesias-Ussel MD, Bowie A, Anderson JG, Li Y, Park LP, Cardona JF, Dennis P, Ebuh V, Geller SA, Jain M, McKenzie MM, Merchant-Borna K, Patel A, Siegel A, Strauss GS, Thoppil JJ, Weinberg AS, Woods CW.2025. Clinical performance of Abbott ID NOW™ COVID-19 2.0 rapid molecular point-of-care test compared to three real-time RT-PCR assays. Microbiol Spectr13:e02033-24. https://doi.org/10.1128/spectrum.02033-24
- ID NOW™ Influenza A & B 2 Product Insert
- ID NOW™ RSV Package Insert

