{question}
{answer}
Please consult the full illustrated instructions included in your kit when taking the test.
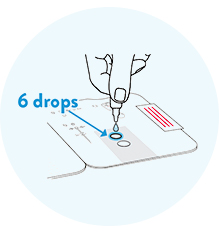
Open the test card and apply six drops to the top hole only.
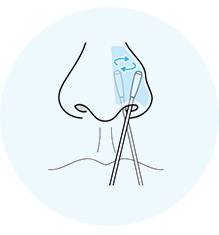
Give both nostrils a shallow swab for about 15 seconds on each side. Big circles – no spinning!
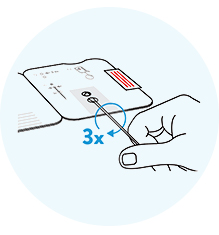
Stick the swab through the
bottom hole into the top hole. Turn the swab to the right clockwise 3x. Fold the card.
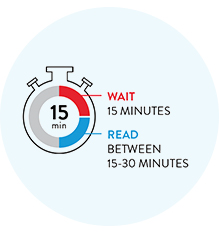
Wait 15 minutes to see your reliable COVID-19 results.
Since the launch of the BinaxNOW™ COVID-19 Antigen Self Test, Abbott continued testing for product stability to extend the expiration date and have shared these results with the FDA. Testing has been completed to support a shelf-life (expiration date) of up to 22 months. For BinaxNOW™ COVID-19 Antigen Self Test, part number 195-160 or 195- 180, with lot numbers less than 230000, kits may have a longer than labeled product expiry date. If your lot number is greater than 230000, it will not be listed in this tool and does not have an expiration extension. All BinaxNOW COVID-19 Antigen Self Test kits currently have a twenty-two-month expiry date.
| KIT LOT NUMBER | ORIGINAL PRINTED EXPIRATION DATE^ | EXTENDED EXPIRATION DATE |
|---|
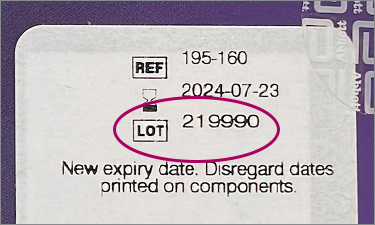
Note: do not use LOT number on individual kit components (test cards, nasal swabs, reagent bottles, instructions, or fact sheet)
Expiration date extension information for other self-test brands can be found at the FDA website:
https://www.fda.gov/medical-devices/coronavirus-covid-19-and-medical-devices/home-otc-covid-19-diagnostic-tests
Disclaimer:
This tool is designed to offer a convenient way for consumers to lookup extended dating by lot number, utilizing information shared by Abbott with the FDA. For official information, please see the FDA letter listing BinaxNOW™ COVID-19 Antigen Self Test kit lot numbers, currently labeled kit expiry and new kit expiry date available on the FDA website at https://www.fda.gov/media/158003/download.
Since the launch of the BinaxNOW™ COVID-19 Antigen Self Test, Abbott continued testing for product stability to extend the expiration date and have shared these results with the FDA. Testing has been completed to support a shelf-life (expiration date) of up to 22 months. For BinaxNOW™ COVID-19 Antigen Self Test, part number 195-160 or 195- 180, with lot numbers less than 230000, kits may have a longer than labeled product expiry date. If your lot number is greater than 230000, it will not be listed in this tool and does not have an expiration extension. All BinaxNOW COVID-19 Antigen Self Test kits currently have a twenty-two-month expiry date.
| KIT LOT NUMBER | ORIGINAL PRINTED EXPIRATION DATE^ | EXTENDED EXPIRATION DATE |
|---|
Expiration date extension information for other self-test brands can be found at the FDA website:
https://www.fda.gov/medical-devices/coronavirus-covid-19-and-medical-devices/home-otc-covid-19-diagnostic-tests
Disclaimer:
This tool is designed to offer a convenient way for consumers to lookup extended dating by lot number, utilizing information shared by Abbott with the FDA. For official information, please see the FDA letter listing BinaxNOW™ COVID-19 Antigen Self Test kit lot numbers, currently labeled kit expiry and new kit expiry date available on the FDA website at https://www.fda.gov/media/158003/download.
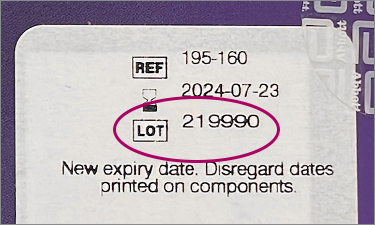
Note: do not use LOT number on individual kit components (test cards, nasal swabs, reagent bottles, instructions, or fact sheet)
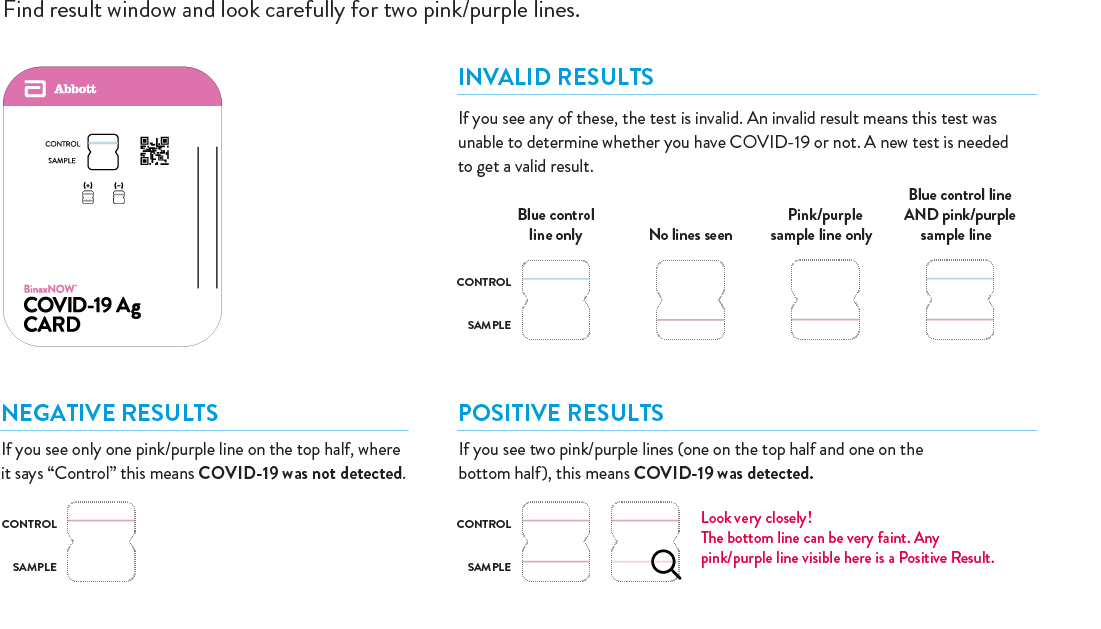
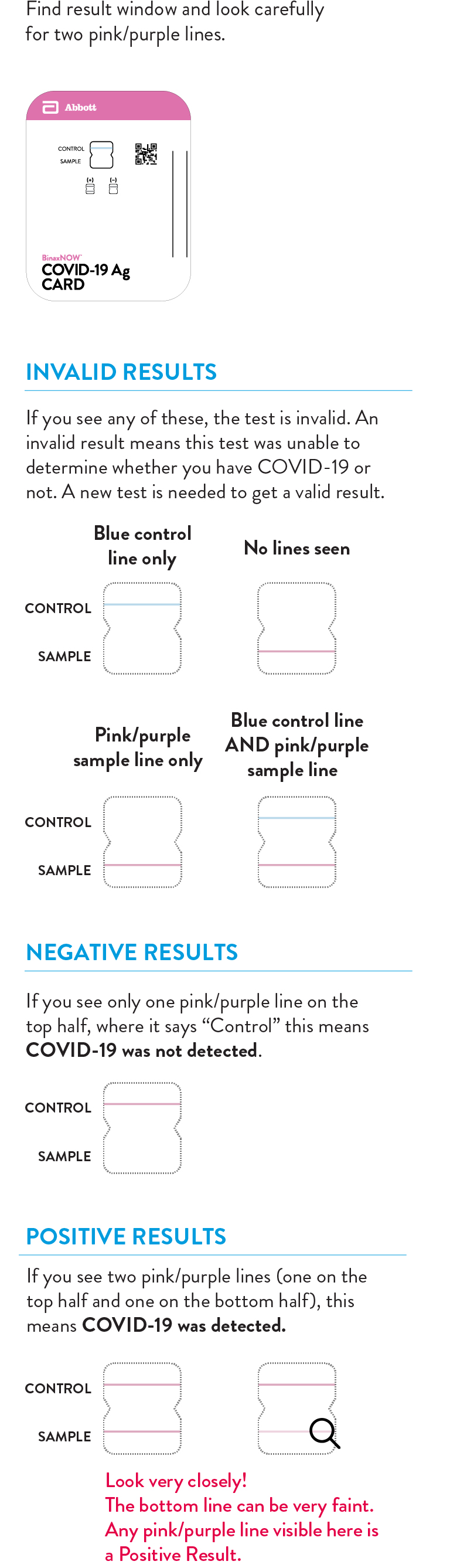
Quick Reference Instructions (as provided in your kit) for BinaxNOW COVID-19 Self Test.
See below for instructions specific to the product you purchased.
Only available in the US.
^Some kits may have an additional label added that states “New expiry date. Disregard dates printed on components.” Original printed expiration date may be covered by this new label.
1. FDA Letter: Revisions Related to Serial (Repeat) Testing for the EUAs of Antigen IVDs. November 1, 2022. Accessed January 26, 2023. https://www.fda.gov/media/162799/download
**Abbott conducted a computational analysis of the detection of multiple SARS-COV-2 strains and predicts no impact to the performance of our BinaxNOW COVID-9 Antigen Self Test.
©2026 Abbott. All rights reserved. Unless otherwise specified, all product and service names appearing in this Internet site are trademarks owned by or licensed to Abbott, its subsidiaries or affiliates. No use of any Abbott trademark, trade name, or trade dress in this site may be made without the prior written authorization of Abbott, except to identify the product or services of the company.
This website is governed by applicable U.S. laws and governmental regulations. The products and information contained herewith may not be accessible in all countries, and Abbott takes no responsibility for such information which may not comply with local country legal process, regulation, registration and usage.
Your use of this website and the information contained herein is subject to our Website Terms and Conditions and Privacy Policy. Photos displayed are for illustrative purposes only. Any person depicted in such photographs is a model. GDPR Statement.
Not all products are available in all regions. Check with your local representative for availability in specific markets. For in vitro diagnostic use only. For i-STAT test cartridge information and intended use, refer to individual product pages or the cartridge information (CTI/IFU) in the i-STAT Support area.
Abbott - A Leader in Rapid Point-of-Care Diagnostics.