Global Point of Care
即时诊断的重要意义



我们的品质故事
观看我们奥斯陆工厂生产线的每个流程、全程保证 Afinion™ 产品的高度准确性与可靠性。



充分利用每一次检测机会
利用 Determine™ HIV Early Detect,检测更多急性 HIV 感染。



Make Every Minute Count
Afinion™ CRP 是一种简单的指尖采血测试,它能在几分钟内提供患者的 CRP 值,有助于减少诊断的不确定性,并指导会诊期间的抗生素使用决策。
特色产品
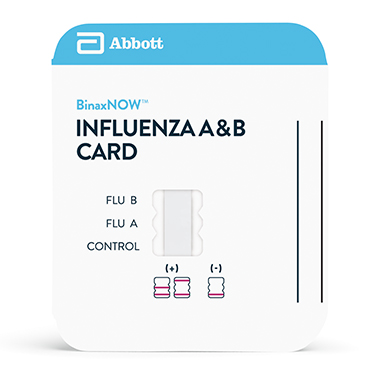
BinaxNOW™ Influenza A&B Card
BinaxNOW™ 甲乙型流感试剂是一种定性检测鼻咽拭子,鼻拭子和鼻洗液/鼻吸出物样本中的甲乙型流感病毒核蛋白抗原的体外免疫层析试剂。
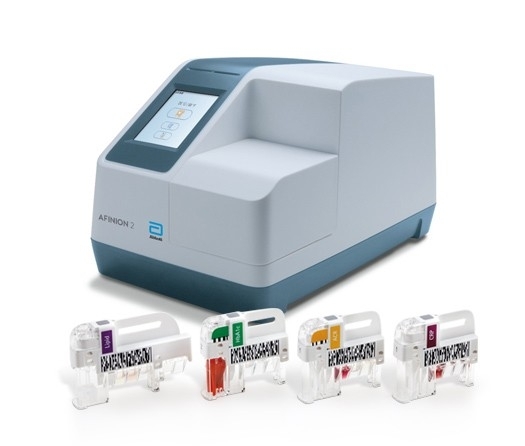
Afinion™ 2
Afinion 2 分析仪是一款紧凑、快速的多项测定分析仪,可即时进行有价值的患者旁检测。只需三个简单的步骤,即可在问诊期间提供 HbA1c、ACR、C 反应蛋白 (CRP) 和血脂组合检测的准确结果。简单的指尖采血检测可在数分钟内提供患者的 HbA1c 值,充分利用问诊过程的每一分钟。
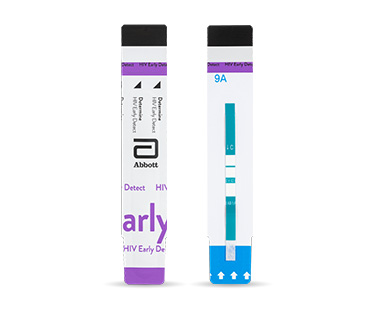
Determine™ HIV
Early Detect
Determine™ HIV Early Detect 可在 20 分钟内提供准确的结果,可以使用指尖全血、静脉全血或血清/血浆进行检测。