Global Point Of Care
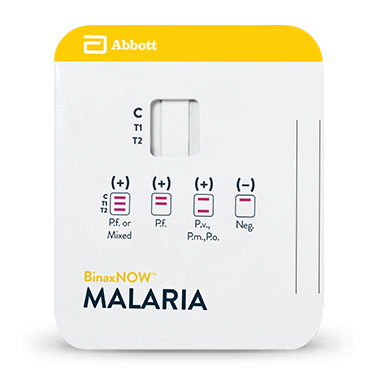
BinaxNOW™ Malaria
Le test BinaxNOW™ Malaria est un test immunochromatographique in vitro conçu pour détecter qualitativement les antigènes circulants de Plasmodium, sur sang total, capillaire ou veineux (prélèvement EDTA) chez les individus présentant les signes et symptômes d’infection paludéenne. Le test cible l’antigène HRP II (Histidine-Rich Protein II, ou protéine riche en histidine II) spécifique du Plasmodium falciparum (P.f.) et un antigène pan-malarique commun aux quatre espèces de Plasmodium pouvant provoquer une infection chez l’être humain : P. falciparum, P. vivax (P.v.), P. ovale (P.o.) et P. malariae (P.m.).
Il est destiné à apporter une aide dans le diagnostic rapide des infections paludéennes humaines et dans le diagnostic différentiel des infections à Plasmodium falciparum (P.f.) afin de les distinguer d’autres infections paludéennes moins virulentes.