Global Point of Care

FLEXIBLE RESPIRATORY
TESTING NOW
WHERE AND WHEN YOU NEED IT
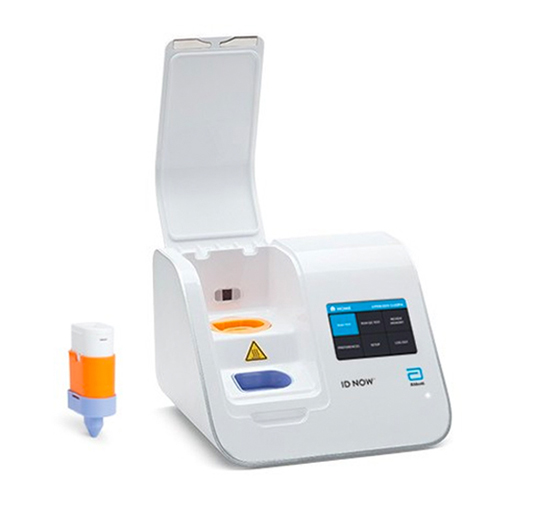
ID NOW™ Provides Rapid Molecular Testing Results for COVID-19, Influenza A & B, RSV or Strep A right at the Point of Care.
Flexible Respiratory Testing Without Compromise NOW
The ID NOW offers full flexibility to test for COVID-19, Influenza A & B, RSV or Strep A on a single platform and with accuracy equivalent to conventional RT-PCR. For even more flexibility, you can run both COVID-19 and Influenza A & B tests using the same patient swab.
With the ID NOW platform, you have the power to decide which tests to run based on patient presentation, circulating prevalence, and seasonality. By reducing unnecessary testing, you can save time and money while increasing diagnostic accuracy in low-prevalence situations.
Learn more about how flexible testing can help to save budget using ID NOW in near patient settings.
Get in touch today to learn more about how you can improve your workflow and potentially save costs with the ID NOW platform and available tests





Respiratory disease testing where you need it NOW
The ID NOW platform can help you to recentralize diagnostic testing around the patient to deliver fast actionable results at any bay or bedside. With a small footprint, the platform can be placed anywhere, allowing near-patient testing or triaging. The availability of bedside testing empowers you to improve patient management. With results being available in just 2-13 minutes, you are empowered to make decisions during the first patient examination.
get answers in minutes where and when you need them to make better patient management decisions
Get in touch today to learn more about how you can improve your workflow and potentially save costs with the ID NOW platform and available tests


Learn how the ID NOW platform can improve patient management with rapid, flexible testing at the patient bedside. Biomedical Scientist Yusuf Gray explains the benefits of testing for multiple respiratory illnesses on one platform.

EASY INTEGRATION WITH MINIMAL ON-DEMAND TRAINING NOW
Designed for intuitive use, the ID NOW platform integrates easily into your workflow and transforms a complex procedure into a simple one. Operational features include on-screen guidance for every step enabling complete ease of use.
ID NOW requires minimal training to operate. And with our online platform myPOCtutor, on-demand training is available anytime, anywhere. So you can stay up-to-date with a wide range of accessible online or face-to-face training courses, assessments, and certifications.
Integrate ID NOW into your daily routine
Get in touch today to learn more about how you can improve your workflow and potentially save costs with the ID NOW platform and available tests


ID NOW is simple to use and helps teams to identify patient pathways quickly. Miriam Boyack, Lead Nurse, Infection Prevention and Control, at Doncaster and Bassetlaw Teaching Hospitals NHS Foundation Trusts shares how easy ID NOW is to use.