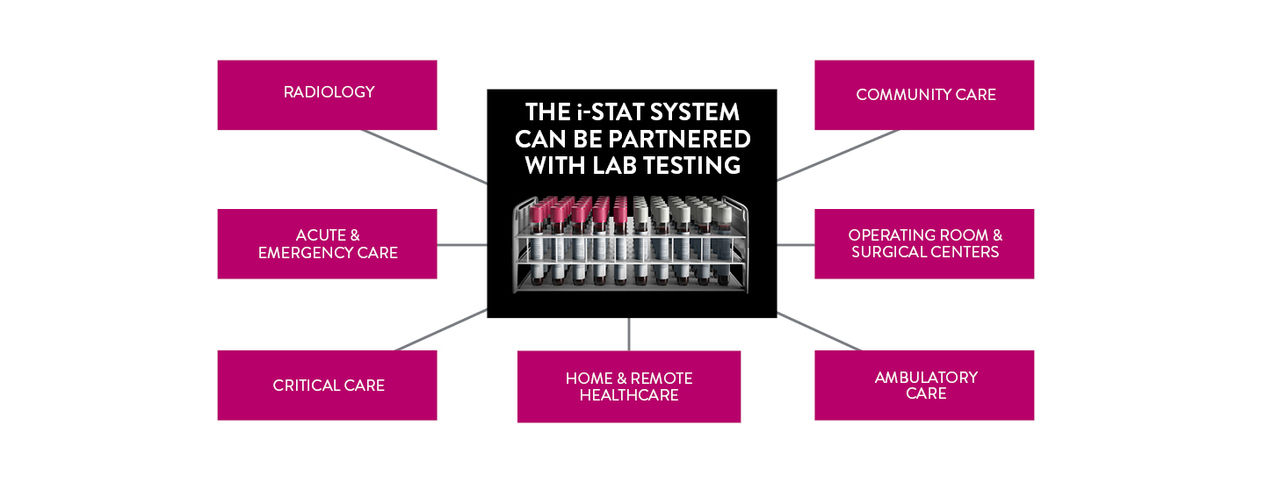
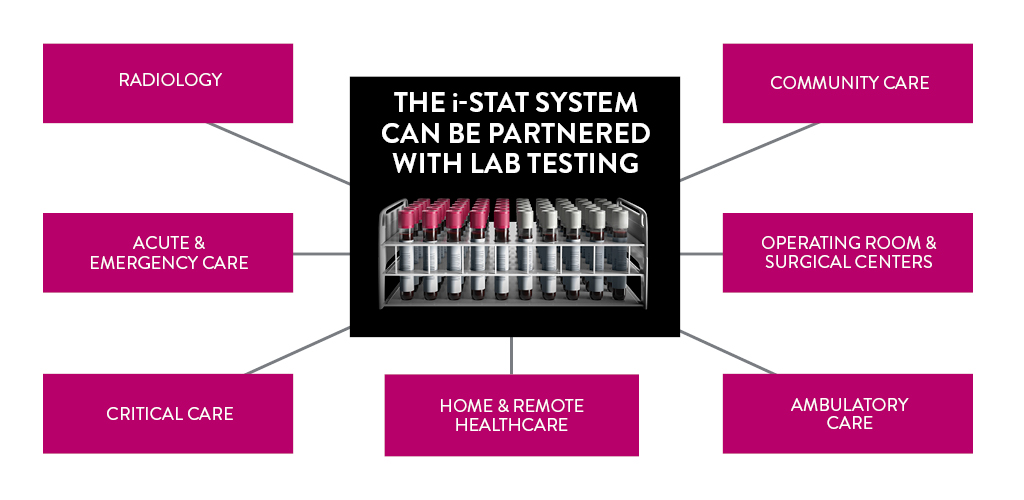
Global point of care



REAL-TIME, LAB-QUALITY RESULTS IN MINUTES
THE i‑STAT SYSTEM DELIVERS DIAGNOSTIC TESTING AND RECORD-KEEPING IN FOUR EASY STEPS, IDEAL FOR USE IN HEALTH CARE LOCATIONS WHERE IT MAY BE DIFFICULT TO GET SAMPLES TO THE CORE LAB
The i-STAT System can help labs meet the demands of and maintain compliance with laboratory regulations, while also increasing efficiency and productivity in the time required to collect, process, and report results. The i-STAT System can't replace the lab; rather, it complements the lab's efforts by providing lab-quality results at the patient's side
Real-time, lab-quality results in minutes
the i‑STAT system delivers diagnostic testing and record-keeping in four easy steps, ideal for use in health care locations where it may be difficult to get samples to the core lab
The i-STAT System can help labs meet the demands of and maintain compliance with laboratory regulations, while also increasing efficiency and productivity in the time required to collect, process, and report results. The i-STAT System can't replace the lab; rather, it complements the lab's efforts by providing lab-quality results at the patient's side







QUALITY WITHIN RANGE
- Each unique i‑STAT System test cartridge contains chemically sensitive biosensors on a silicon chip that is configured for specific analytes
- Quality checks of sample integrity, sensors, and fluidics are automatic with each i-STAT test cartridge, providing confidence and advanced performance
- Liquid quality control can be seamlessly integrated into the testing process by customizable user lockout, ensuring compliance with quality systems
- Broad test menu covers the most commonly used tests in one platform
QUALITY WITHIN RANGE
i-STAT System single-use test cartridges are designed to help reduce the problems multi-use systems face with poor quality and/or clotted samples
- Each unique i‑STAT System test cartridge contains chemically sensitive biosensors on a silicon chip that is configured for specific analytes
- Quality checks of sample integrity, sensors, and fluidics are automatic with each i-STAT test cartridge, providing confidence and advanced performance
- Liquid quality control can be seamlessly integrated into the testing process by customizable user lockout, ensuring compliance with quality systems
- Broad test menu covers the most commonly used tests in one platform



optimize testing
hospital-wide testing priorities during peak infection phases & recommended steps



understaffed labs strained by higher test volume
EXPANDED TESTING CAPACITY
- Reduce exposure
- Alleviate burden on the lab
- Conserve lab resources for an increase in testing volume and COVID-19 diagnoses
lab specimen hand-offs & transport adds risk to care teams and patients
LAB SAFETY TESTING
- Minimize movement of materials and personnel throughout the hospital or between care centers
- Minimize sample handling
- Reduce entry and exit from infectious disease/isolation units to minimize PPE utilization
covid-19 patients need frequent monitoring to assess status changes
diagnostic test use models
- Access timely lab results to monitor and assess patient status across ICU and stable COVID-19 positive patients
supporting your lab-testing priorities
i‑STAT Portable, Handheld Analyzer
- REDUCES BURDEN ON LAB WITH NURSE-DRIVEN BEDSIDE TEST PROTOCOLS
- ELIMINATES NEED TO TRANSPORT SAMPLES OR LEAVE ISOLATION AREAS
- EASILY WIPES CLEAN TO DECONTAMINATE
learn how abbott can support your hospital-wide priorities




supporting your lab-testing priorities
i‑STAT Portable, Handheld Analyzer
- REDUCES BURDEN ON LAB WITH NURSE-DRIVEN BEDSIDE TEST PROTOCOLS
- ELIMINATES NEED TO TRANSPORT SAMPLES OR LEAVE ISOLATION AREAS
- EASILY WIPES CLEAN TO DECONTAMINATE
learn how abbott can support your hospital-wide priorities
supporting your lab-
testing priorities
i‑STAT Portable, Handheld
Analyzer
- Reduces burden on lab with
Nurse-driven bedside test
protocols - Eliminates need to transport
Samples or leave isolation
areas - Easily wipes clean to
Decontaminate
learn how abbott can
support your hospital-wide
priorities
KEY i-STAT TEST CARTRIDGES
- Provide fast results which can assist in the early identification of deteriorating health in patients and can help to identify patients that are critically ill
- Single-use, self-contained cartridges to reduce infection risk by limiting exposure
CONTINUED USER SUPPORT
In addition to resources found in our web site's Support area, regularly scheduled, live, online training sessions are offered with instructions on how to use the i-STAT System.



increase compliance efficiency and oversight

The i-STAT System is designed to meet laboratory accrediting organizations (and CLIA) requirements for with-patient testing.
Adoption of with-patient testing can help alleviate pressure on lab technicians by reducing the amount of test requests and telephone call-back procedures, thereby freeing them to focus on other more complex testing.
The i-STAT System is designed to meet laboratory accrediting organizations (and CLIA) requirements for with-patient testing.
References
- Ismail F et al. Scand J Trauma Resusc Emerg Med 2015; 23:68-75.
- Falter F. Benefits of Handheld Diagnostics in the CVOR. Point-of-Care Testing Supplement - Hospital Healthcare Europe, August 2015.





