global Point Of Care
Identify COVID-19 contagious patients, in 15 minutes, with patient friendly nasal sample collection
Panbio COVID-19 Ag Rapid test


Panbio™ COVID-19 Self-Test Receives CE Mark Increasing
Access To Fast, Reliable Testing in the United Kingdom
Panbio™ COVID-19 Self-Test Receives CE Mark Increasing
Access To Fast, Reliable Testing in the United Kingdom
For people suspected of COVID-19 exposure
Panbio™ COVID-19 Ag rapid test device offers:
High performance compared to nasal pcr
- Panbio™ vs. Nasal PCR: Sensitivity 98.1%, Specificity 99.8%
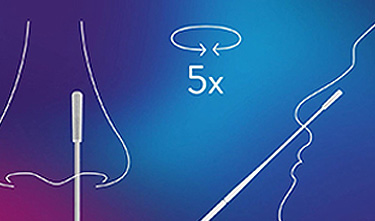
Patient-friendly nasal* sample collection
- ~ 2 cm insertion depth minimises undesirable reflexes like coughing or sneezing1
- Patient-friendly nasal self-collected swab option minimises health worker exposure
Fast indentification of contagious individuals
- Test results in 15 minutes
Accessible, large-scale testing that helps contain the virus spread
- Enabling immediate treatment or isolation measures to minimise transmission
Panbio™ COVID-19 Ag rapid test device can be used to, in all ages:
- Screen Asymptomatic individuals
- Test in individuals presenting with symptoms in the last 7 days
- Test individuals suspected of exposure to COVID-19
Identify potentially contagious patients with the Panbio™ COVID-19 Ag Rapid Test
helpful information
Panbio™ COVID-19 Ag rapid test device nasal swab procedure
Learn more about how to perform a nasal swab in this animation.
Panbio™ COVID-19 Ag rapid test device nasal swab procedure
Learn more about how to perform a nasal swab in this live action video.

Product Brochure
Learn more about the performance of the Panbio™ COVID-19 Ag Rapid Test Device.
References
1 Pondaven-Letourmy S, et al. European Annals of Otorhinolaryngology, Head and Neck Diseases. 2020.
* PANBIO™ COVID-19 Ag rapid test device ™ is also available as a nasopharyngeal kit. See the nasophayngeal instructions for use here. See the product sheet here and learn more about the test procedure here.
Panbio™ COVID-19 Ag Rapid Test Device is an in vitro diagnostic rapid test for the qualitative detection of SARS-CoV-2 antigen (Ag) in human nasal swab specimens from individuals who meet COVID-19 clinical and / or epidemiological criteria. Panbio™ COVID-19 Ag Rapid Test Device is for professional use only and is intended to be used as an aid in the diagnosis of SARS-CoV-2 infection. The product may be used in any laboratory and non-laboratory environment that meets the requirements specified in the Instructions for Use and local regulation. The test provides preliminary test results. Negative results don’t preclude SARSCoV-2 infection and they cannot be used as the sole basis for treatment or other management decisions. Negative results must be combined with clinical observations, patient history, and epidemiological information. The test is not intended to be used as a donor screening test for SARS-CoV-2.
Download the full IFU from here.