Global Point of Care


Empowering LGUs in Dengue and Hepatitis programs
An integrated approach project for the devolution of Dengue, Hepatitis B and Hepatitis C in the Philippines
Dengue and Hepatitis: Key Public Health Concerns
Dengue and Hepatitis are two of the most important diseases in the country, both at the local and national government level. In the Devolution Transition Plan issued by Department of Health (DOH), these two programs are now devolved to Local Government Units (LGUs).1
DENGUE
Dengue is probably the most well-known and feared tropical disease in our country.
The first recorded dengue epidemic in Southeast Asia occurred in Manila in 1954, and dengue has since remained endemic.2 In the latest report, there was an observed 30% increase in the number of dengue cases after the start of the rainy season. There were also seven regions that remain on high alert due to the spike in the six weeks before June 29: National Capital Region (NCR), CALABARZON, MIMAROPA, Cagayan Valley, Western Visayas, Ilocos Region and Central Luzon.3
Rapid testing is critical for early diagnosis of dengue infection. While dengue fever may be diagnosed clinically, use of rapid test helps secure a definitive diagnosis.4
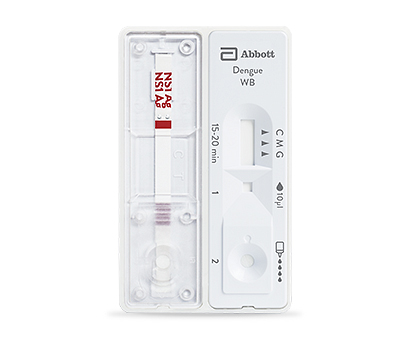
Explore our diagnostic tools for rapid results and comprehensive detection in the fight against dengue.

Diagnose and treat dengue quickly with rapid testing.
hepatitis
Hepatitis is also a major public health concern in the Philippines and many of those who have the disease may not even know they have it until it’s too late.
Around one in 10 people have chronic hepatitis B, and six in 1,000 have chronic hepatitis C. To eliminate this deadly disease, we must invest in hepatitis testing and treatment services.
Aside from a program like the Devolution Transition Plan being the right thing to do for people’s health, it is also a very wise investment, as it can save money by avoiding much more costly care for liver cancer and cirrhosis.5
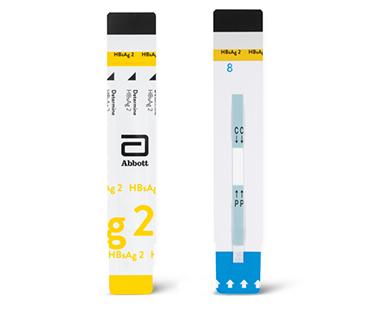
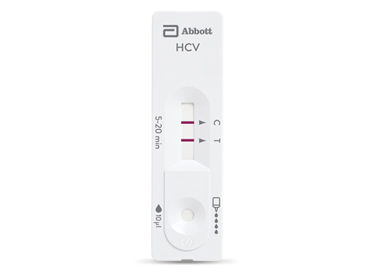
Learn how our solutions can help in the early identification and effective management of hepatitis B and C, contributing to global efforts to eliminate these diseases.

Diagnose and treat hepatitis quickly with rapid testing.
References
- Devolution Transition Plan 2022 – 2024. Department of Health, Philippines
- Perspectives and lessons from the Philippines’ decades-long battle with dengue. Ong, E at al. The Lancet Regional Health - Western Pacific 2022;24: 100505
- https://www.philstar.com/headlines/2024/07/17/2370703/dengue-cases-30. Accessed July 18, 2024
- Definitive tests for dengue fever: when and which should I use? Singapore Med J 2017; 58(11): 632-635
- https://www.who.int/philippines/news/commentaries/detail/ph-must-act-to-eliminate-hepatitis accessed July 19, 2024