ASSESSING CHALLENGING
TBI CASES
TBI CASES
Dr. Davlantes discusses the types of cases he sees in his ED that present challenges to assessing possible head injury at the point of care.
Global point of care
The i-STAT TBI cartridge* is the first point-of-care venous whole blood test for mild TBI that measures brain-specific biomarkers and delivers objective data in 15 minutes.1
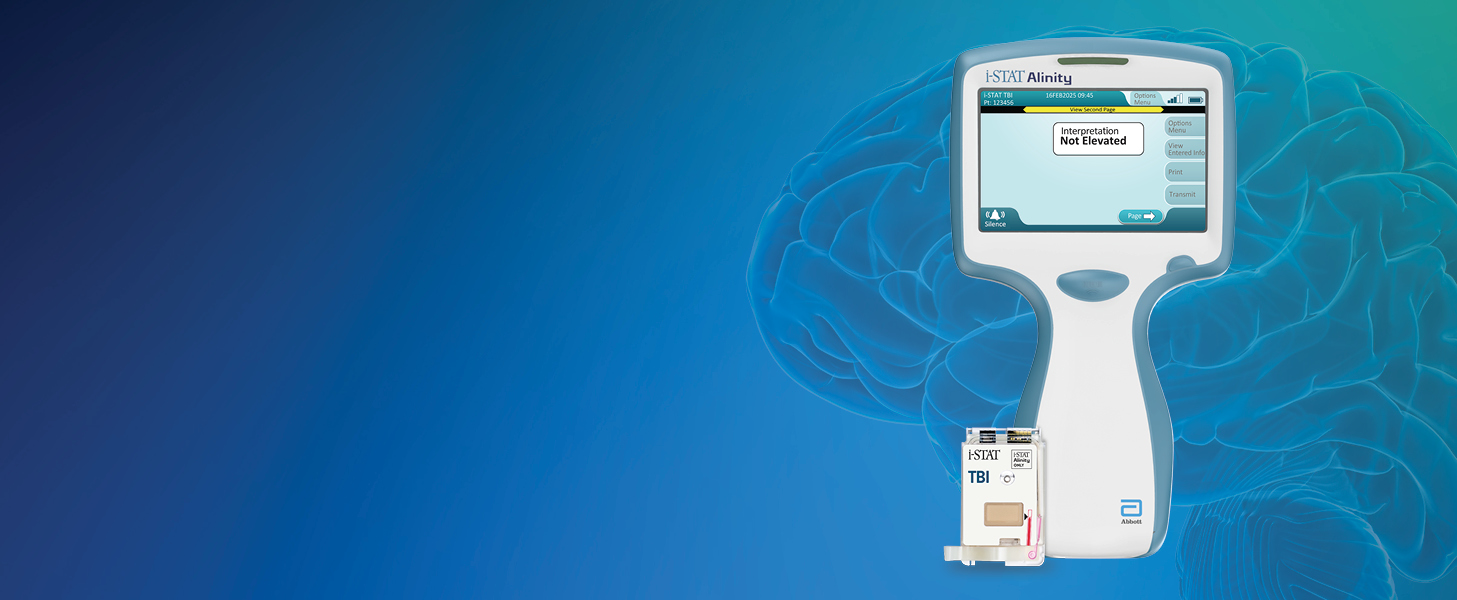

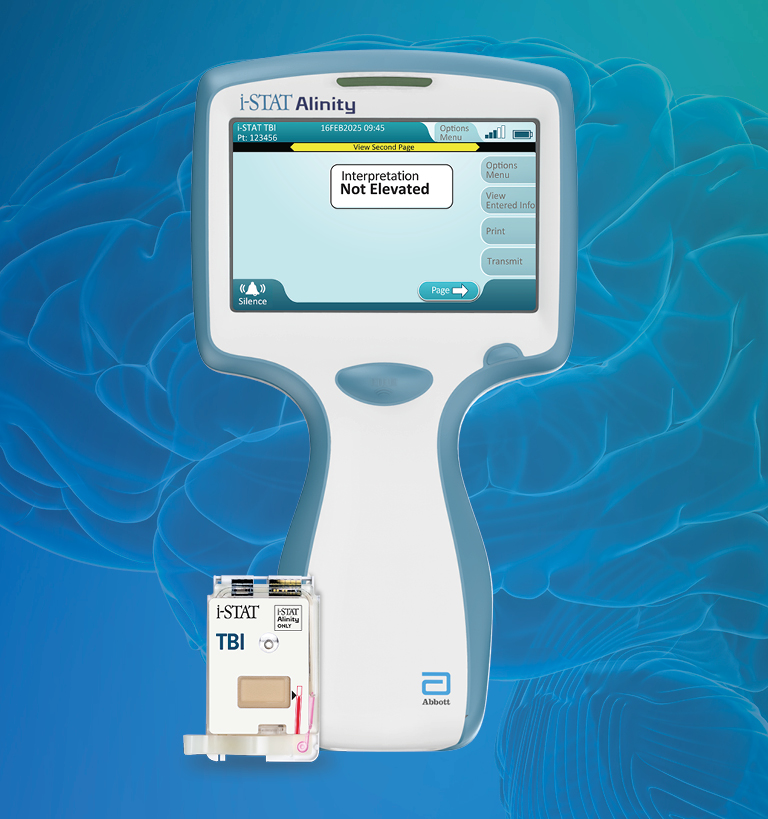
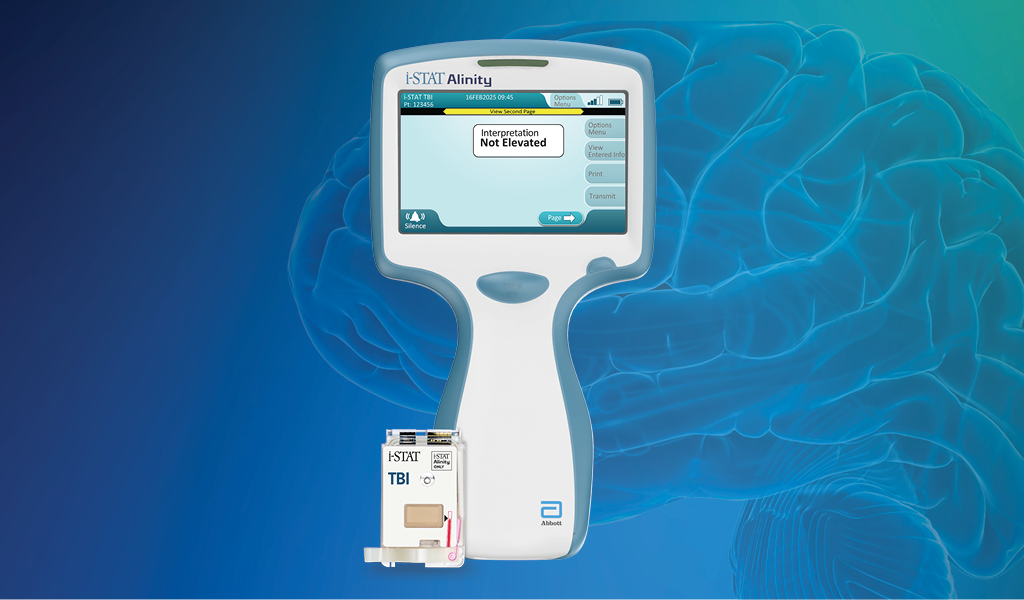
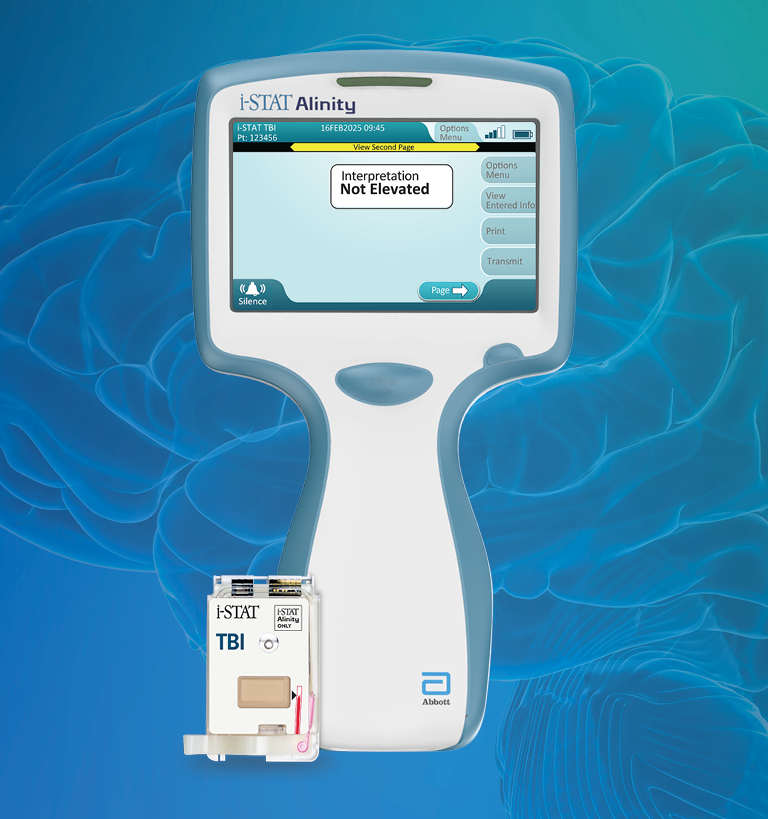
The i-STAT TBI cartridge* is the first point-of-care venous whole blood test for mild TBI that measures brain-specific biomarkers and delivers objective data in 15 minutes.1
What impact will the i-STAT TBI cartridge have on your patients and care team? Contact us and explore our innovative, point-of-care products that deliver lab-quality results in minutes.




What impact will the i-STAT TBI cartridge have on your patients
and care team?

This white paper explains more about the role biomarkers play in the assessment of mild traumatic brain injury.

Discover more on the role of biomarkers (GFAP and UCH-L1) in assessing mild traumatic brain injury (mTBI).
A leader in rapid point-of-care diagnostics.
©2024 Abbott. All rights reserved. Unless otherwise specified, all product and service names appearing in this Internet site are trademarks owned by or licensed to Abbott, its subsidiaries or affiliates. No use of any Abbott trademark, trade name, or trade dress in this site may be made without the prior written authorization of Abbott, except to identify the product or services of the company.
This website is governed by applicable U.S. laws and governmental regulations. The products and information contained herewith may not be accessible in all countries, and Abbott takes no responsibility for such information which may not comply with local country legal process, regulation, registration and usage.
Your use of this website and the information contained herein is subject to our Website Terms and Conditions and Privacy Policy: US Citizens | Non-US Citizens. Photos displayed are for illustrative purposes only. Any person depicted in such photographs is a model. GDPR Statement
Not all products are available in all regions. Check with your local representative for availability in specific markets. For in vitro diagnostic use only. For i-STAT test cartridge information and intended use, refer to individual product pages or the cartridge information (CTI/IFU) in the i-STAT Support area.
Abbott - A Leader in Rapid Point-of-Care Diagnostics.