Global point of care
OVERCOMING CHALLENGES IN THE EVALUATION OF SUSPECTED MILD TRAUMATIC BRAIN INJURY (mTBI)
With the i-STAT TBI Plasma test for use with
the i-STAT ALINITY System
the i-STAT ALINITY System
The i-STAT TBI Plasma test is not intended for use as a point-of-care-device



CURRENT METHODS FOR EVALUATING SUSPECTED mTBI CAN BE SUBJECTIVE
Millions of patients undergo emergency department (ED) evaluation for mTBI each year. But the Glasgow Coma Scale (GCS) score and clinical evaluation only provide subjective information, leading many to use CT.
The i-STAT TBI Plasma test may be the solution to time-consuming and often unnecessary head CTs for mTBI.1
The Drawbacks of CT
CT FOR TBI HAS A LOW DIAGNOSTIC YIELD - AND SIGNIFICANT DRAWBACKS


Clinical decision rules (CDRs), such as the Canadian CT Head Rule have had limited impact on the number or diagnostic yield of CT for the evaluation of mTBI3-5
An estimated 82% of patients with TBI undergo CT, but >90% show no evidence of traumatic abnormality1
A systematic review of 14 studies found that among patients with minor head trauma, only 7% had severe intracranial injuries identified on a CT, while <1% had injuries that required neurosurgical intervention2
AS A TOOL FOR EVALUATING SUSPECTED mTBI, CT HAS SIGNIFICANT DRAWBACKS
- The time from ordering to reading CT can be up to 3 hours—about half the total time for evaluation of mTBI6
- Organ radiation doses from CT scanning are substantially higher than those from conventional radiography7
- The radiation dose of head CT scan is equivalent to 100 times that of a chest x-ray
- Difficult-to-assess patients, such as those who are intoxicated, present a challenge
Biomarkers for mTBI
BIOMARKERS OF BRAIN INJURY HAVE THE POTENTIAL TO REDUCE THE AMOUNT OF UNNECESSARY CT PERFORMED FOR SUSPECTED mTBI
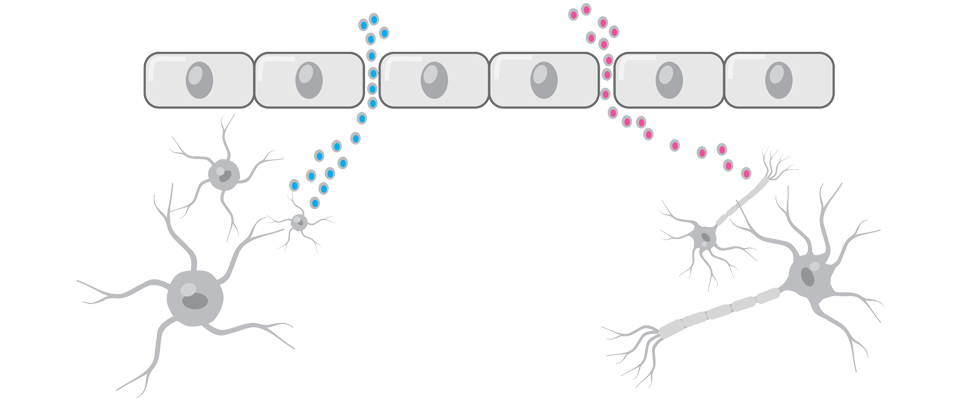
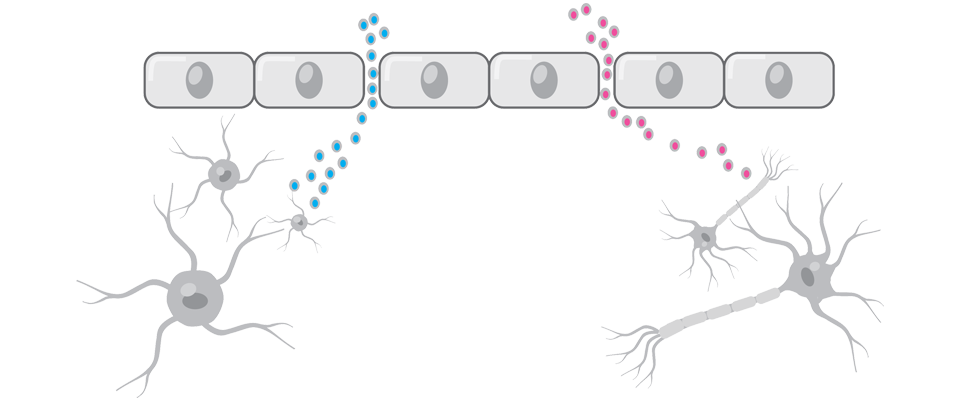

FOLLOWING TBI, THERE IS INCREASED PERMEABILITY AND LEAKAGE OF MOLECULES ACROSS THE BLOOD-BRAIN BARRIER8,9
Brain proteins that cross the blood-brain barrier when it’s disrupted can serve as biomarkers for TBI.8
WHILE SEVERAL BIOMARKERS OF TBI HAVE BEEN STUDIED, TO HAVE DIAGNOSTIC VALUE IN THE ED, CERTAIN CHARACTERISTICS ARE CONSIDERED ESSENTIAL10:
- Readily measurable in an accessible biofluid, such as peripheral blood, shortly after injury
- Elevated peripheral blood levels specific to brain injury
- Sensitivity high enough to assess mild injury
THE i-STAT TBI Plasma Test
INTRODUCING THE i-STAT TBI PLASMA test, A BIOMARKER-BASED ASSAY DESIGNED TO OBJECTIVELY ASSESS THE NEED FOR CT


The i-STAT TBI Plasma test combines 2 assays for brain-specific biomarkers, glial fibrillary acidic protein (GFAP) and ubiquitin carboxyl-terminal hydrolase L1 (UCH-L1), in a single multiplex test designed to help determine the need for
head CT.11
INTENDED USE11
The interpretation of test results is used, in conjunction with other clinical information, to aid in the evaluation of patients, 18 years or older, presenting with suspected mTBI (GCS score 13-15) within 12 hours of injury to assist in determining the need for head CT.*
*The i-STAT TBI Plasma test is to be used with plasma prepared from ethylenediaminetetraacetic acid (EDTA)–anticoagulated specimens in clinical laboratory settings by a healthcare professional. It is not intended for point-of-care use.
THE i-STAT TBI PLASMA TEST REDUCES UNCERTAINTY BY PROVIDING A QUANTIFIABLE, OBJECTIVE ASSESSMENT TO INFORM EVALUATION OF SUSPECTED mTBI
- With a negative predictive value (NPV) of 99.3% in the pivotal study, i-STAT TBI Plasma Test can decrease reliance on CT imaging, potentially minimizing unnecessary radiation exposure, length of stay, and resource utilization for patients who are not at risk of abnormality on CT11
- The high clinical sensitivity and NPV of the i-STAT TBI Plasma Test provide confidence in aiding decisions for the safe discharge of patients without performing CT

*The i-STAT TBI Plasma test is to be used with plasma prepared from EDTA-anticoagulated specimens in clinical laboratory settings by a healthcare professional. It is not intended for point-of-care use.
WANT TO KNOW MORE ABOUT THE i-STAT ALINITY SYSTEM?
*The i-STAT TBI Plasma test is to be used with plasma prepared from EDTA-anticoagulated specimens in clinical laboratory settings by a healthcare professional. It is not intended for point-of-care use.
The i-STAT TBI PLASMA TEST HAS THE POTENTIAL TO REDUCE THE NUMBER OF UNNECESSARY CT FOR SUSPECTED mTBI by up to 40%
40.4% of negative results in the pivotal study had a true negative result on the i-STAT TBI Plasma test — suggesting a potential reduction in unnecessary CT of up to 40%11,12



REDUCING THE AMOUNT OF CT MAY HAVE POSITIVE IMPLICATIONS FOR ED WORKFLOW, RESOURCE UTILIZATION, AND PATIENT SATISFACTION
For each scan avoided, associated wait times, resource utilization, and costs may also be eliminated
The 15-Minute Instrument Time* of i-STAT TBI Plasma Test May Provide Important Benefits:
Shorter wait times
Rapid Reassurance
Timely Discharge
What impact could a biomarker assay that can objectively and accurately aid in ruling out the need for CT in the evaluation of mTBI have on your ED?
Biomarkers Behind the Test
GFAP AND UCH-L1 ARE WELL-VALIDATED, COMPLEMENTARY, BRAIN-SPECIFIC BIOMARKERS RELEASED INTO THE BLOODSTREAM FROM 2 DIFFERENT CELL TYPES FOLLOWING Traumatic brain injury
GFAP IS A STRUCTURAL PROTEIN FOUND ALMOST EXCLUSIVELY IN ASTROCYTES13
- GFAP is a specific marker of astrocyte injury in either white or gray matter that is elevated in patients with traumatic intracranial abnormalities on CT11,14
- GFAP reliably distinguishes between trauma patients with mTBI and those without head injury15
- Unlike some previously studied biomarkers, GFAP levels are not affected by extracranial trauma or exercise16-19
UCH-L1 IS A DEGRADATION ENZYME HIGHLY AND EXCLUSIVELY EXPRESSED IN NEURONS13
- Blood levels have been demonstrated to distinguish mTBI patients from those without injuries20
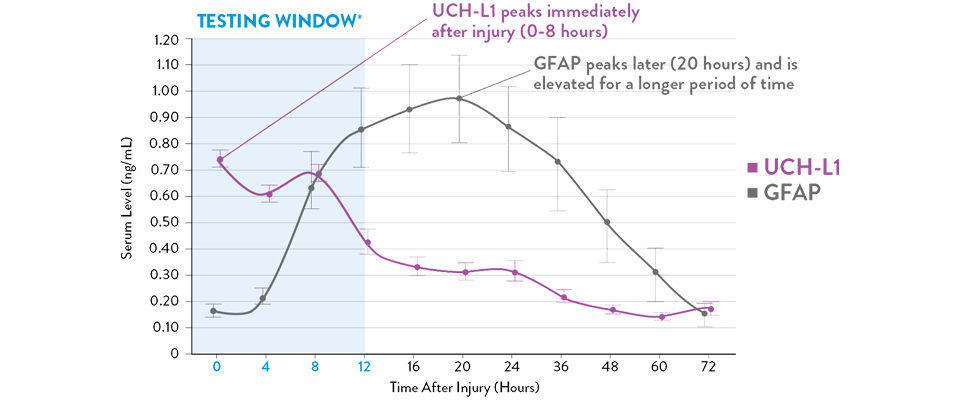
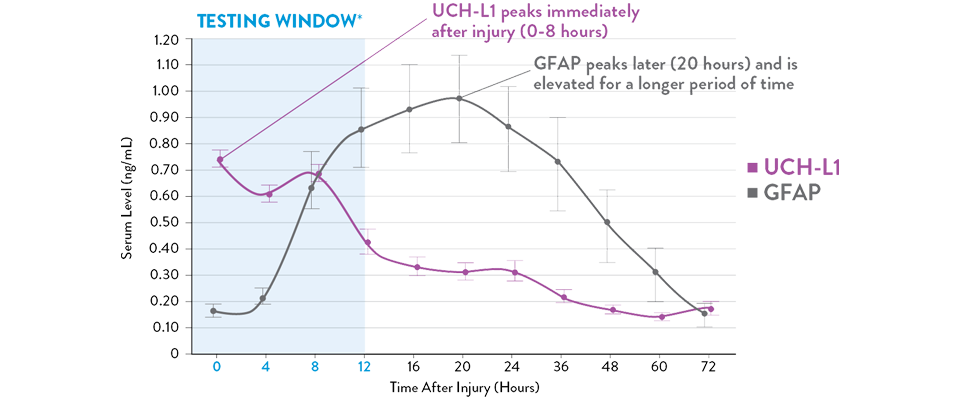

Adapted from Papa et al, 2016
Serum UCH-L1 levels peak 0 to 8 hours post injury and steadily decrease over 48 hours while GFAP peaks at 20 hours and declines slowly 72 hours after brain injury.21 The i-STAT TBI Plasma Test measures levels of both biomarkers during the optimal 12-hour period following injury.11
*The i-STAT TBI Plasma Test is to be used with plasma prepared from EDTA-anticoagulated specimens in clinical laboratory settings by a healthcare professional. It is not intended for point-of-care use.
References:
1. Korley FK, Kelen GD, Jones CM, Diaz-Arrastia R. Emergency department evaluation of traumatic brain injury in the United States, 2009-2010. J Head Trauma Rehabil. 2016;31(6):379-387.
2. Easter JS, Haukoos JS, Meehan WP, Novack V, Edlow JA. Will neuroimaging reveal a severe intracranial injury in this adult with minor head trauma? The Rational Clinical Examination Systematic Review. JAMA. 2015;314(24):2672-2681.
3. Sharp AL, Nagaraj G, Rippberger EJ, et al. Computed tomography use for adults with head injury: describing likely avoidable emergency department imaging based on the Canadian CT Head Rule. Acad Emerg Med. 2017;24(1):22-30.
4. Sultan HY, Boyle A, Pereira M, Antoun N, Maimaris C. Application of the Canadian CT head rules in managing minor head injuries in a UK emergency department: implications for the implementation of the NICE guidelines. Emerg Med J. 2014;21(4):420-425.
5. Stiel IG, Clement CM, Grimshaw JM, et al. A prospective cluster-randomized trial to implement the Canadian CT Head Rule in emergency departments. CMAJ. 2010;182(14):1527-1532.
6. Michelson EA, Huff JS, Loparo M, et al. Emergency department time course for mild traumatic brain injury workup. West J Emerg Med. 2010;19(4):635-640.
7. US Food and Drug Administration. What are the radiation risks from CT? Updated December 5, 2017. Accessed November 20, 2020. https://www.fda.gov/radiation-emitting-products/medical-x-ray-imaging/what-are-radiation-risks-ct.
8. Zetterberg H, Blennow K. Fluid biomarkers for mild traumatic brain injury and related conditions. Nat Rev Neurol. 2016;12(10):563-574.
9. Chodobski A, Zink BJ, Symydynger-Chodobska J. Blood-brain barrier pathophysiology in traumatic brain injury. Transl Stroke Res. 2011;2(4):492-516.
10. Wang KK, Yang Z, Zhu T, et al. An update on diagnostic and prognostic biomarkers for traumatic brain injury. Expert Rev Mol Diagn. 2018;18(2):165-180.
11. i-STAT TBI Plasma Cartridge. Instructions for use. Abbott Point of Care Inc. Abbott Park, IL; 2020.
12. Data on file. Abbott Point of Care Inc.
13. Diaz-Arrastia R, Wang KKW, Papa L, et al; TRACK-TBI Investigators. Acute biomarkers of traumatic brain injury: relationship between plasma levels of ubiquitin C-terminal hydrolase-L1 and glial fibrillary acidic protein. J Neurotrauma. 2014;31(1):19-25.
14. Metting Z, Wilczak N, Rodiger LA, Schaaf JM, van der Naalt J. GFAP and S100B in the acute phase of mild traumatic brain injury. Neurology. 2012;78(18):1428-1433.
15. Papa L, Lewis LM, Silvestri S, Falk JL, Giordano P, Brophy GM. Serum levels of Ubiquitin C-terminal hydrolase (UCH-L1) distinguish mild traumatic brain injury (TBI) from trauma controls and are elevated in mild and moderate TBI patients with intracranial lesions and neurosurgical intervention. J Trauma Acute Care Surg. 2012;72(5):1335-1344.
16. Jones A, Jarvis P. Review of the potential use of blood neuro-biomarkers in the diagnosis of mild traumatic brain injury. Clin Exp Emerg Med. 2017;4(3):121-127.
17. Schulte S, Podlog LW, Hamson-Utley JJ, Strathmann FG, Strϋder HK. A systematic review of the biomarker S100B: implications for sport-related concussion management. J Athl Train. 2014;49(6):830-850.
18. Steiner J, Bernstein H-G, Bielau H, et al. Evidence for a wide extra-astrocytic distribution of S100B in human brain. BMC Neurosci. 2007;8:2.
19. Pelinka LE, Kroepfl A, Schmidhammer R, et al. Glial fibrillary acidic protein in serum after traumatic brain injury and multiple trauma. J Trauma. 2004;57(5):1006-1012.
20. Papa L, Lewis LM, Falk JL, et al. Elevated levels of serum glial fibrillary acidic protein breakdown products in mild and moderate traumatic brain injury are associated with intracranial lesions and neurosurgical intervention. Ann Emerg Med. 2012;59(6):471-483.
21. Papa L, Brophy GM, Welch RD, et al. Time course and diagnostic accuracy of glial and neuronal blood biomarkers GFAP and UCH-L1 in a large cohort of trauma patients with and without mild traumatic brain injury. JAMA Neurol. 2016;73(5):551-560.

