Play the Demo
Learn how DIGIVAL works by playing our interactive product demonstration.
Global point of care
Sign up now to receive clinical data and learn how DIGIVAL can increase the quality standards of your lab.
It can lead to a faster and more precise diagnosis—and help to optimize the management of patients and their diseases.1 Using technology to read your diagnostic testing helps your team maintain trust with the caregivers who count on your lab.



It can lead to a faster and more precise diagnosis—and help to optimize the management of patients and their diseases.1 Using technology to read your diagnostic testing helps your team maintain trust with the caregivers who count on your lab.



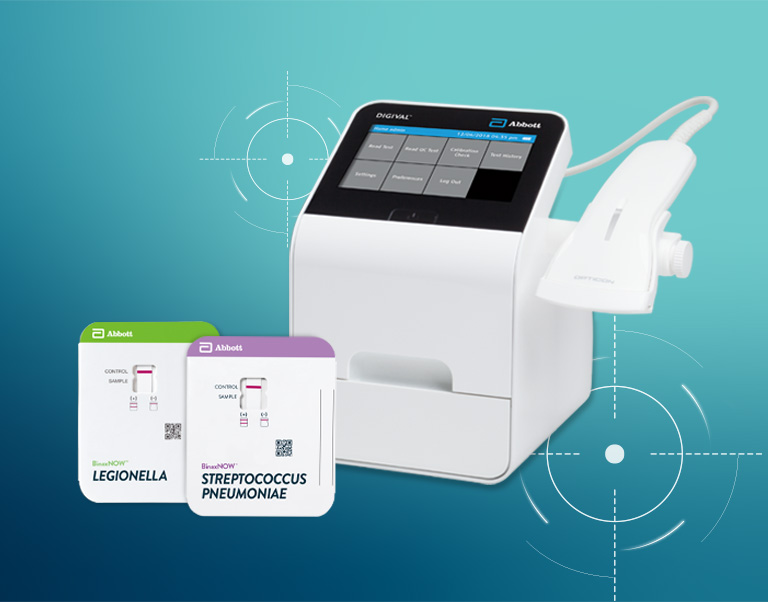
DIGIVAL is a camera-based instrument that helps laboratory technicians produce objective results in seconds. This automated technology accurately detects and identifies BinaxNOW S. pneumoniae and Legionella results.


Objective results—Eliminate operator subjectivity and provide consistency in the reading of lateral flow test assays.5
Rapid Results—Combine the control of a connected instrument with the speed of a rapid lateral flow assay.5
Easy to use —Scan the barcode, enter the patient ID, and place the test into DIGIVAL.5
Flexible access—Save, transfer to HIS/LIMS and print results directly from DIGIVAL.5
Enhanced Quality Control—Empower QC module and lockout feature.5
Barcode Technology—Maximize accuracy and minimize human error. The barcode scanner allows the user to quickly enter and connect patient ID and test information.5
Workflow flexibility—Experience flexible implementation into existing workflow5. Choose between reading only or fully automated mode to match your needs.




Adhering to Good Lab Practices (GLP) and quality control is essential to demonstrating the data you are submitting is accurate, and valid. DIGIVAL offers several ways to prevent human error from the process.
this feature ensures QC tests are run every time your lab receives a new batch of product.
allows the laboratory to record and objectively monitor the performances of the technicians.
DIGIVAL provides all the data you need to satisfy training compliance.



DIGIVAL will capture, interpret and transmit results of select Abbott rapid lateral flow tests and can be used in the laboratory and point of care settings. It is a camera based instrument that will detect the presence of and identify a completed lateral flow assay, analyze the intensity of the test and control line and display the results (positive, negative or invalid) on the color touch screen.

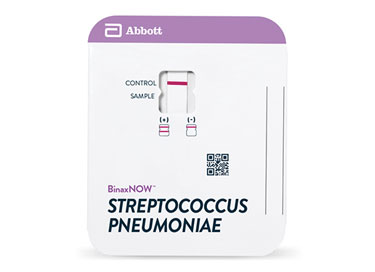

BinaxNOW S. pneumoniae Urinary Antigen Card is a rapid assay for the qualitative detection of S. pneumoniae antigen in the urine of patients with pneumonia and in the cerebral spinal fluid (CSF) of patients with meningitis. It is intended to aid, in conjunction with culture and other methods, in the diagnosis of both pneumococcal pneumonia and pneumococcal meningitis.



BinaxNOW Legionella Urinary Antigen Card is a rapid assay for the qualitative detection of Legionella pneumophila serogroup 1 antigen in urine samples from patients with symptoms of pneumonia. It is intended to aid in the presumptive diagnosis of Legionnaires’ disease caused by L. pneumophila serogroup 1 in conjunction with culture and other methods.
Learn how DIGIVAL works by playing our interactive product demonstration.
Learn how the BinaxNOW Legionella Urinary Antigen Card works by playing our interactive product demonstration.
Learn how to use the BinaxNOW Streptococcus pneumoniae Urinary Antigen Card by watching this product demonstration.
©2026 Abbott. All rights reserved. Unless otherwise specified, all product and service names appearing in this Internet site are trademarks owned by or licensed to Abbott, its subsidiaries or affiliates. No use of any Abbott trademark, trade name, or trade dress in this site may be made without the prior written authorization of Abbott, except to identify the product or services of the company.
This website is governed by applicable U.S. laws and governmental regulations. The products and information contained herewith may not be accessible in all countries, and Abbott takes no responsibility for such information which may not comply with local country legal process, regulation, registration and usage.
Your use of this website and the information contained herein is subject to our Website Terms and Conditions and Privacy Policy. Photos displayed are for illustrative purposes only. Any person depicted in such photographs is a model. GDPR Statement.
Not all products are available in all regions. Check with your local representative for availability in specific markets. For in vitro diagnostic use only. For i-STAT test cartridge information and intended use, refer to individual product pages or the cartridge information (CTI/IFU) in the i-STAT Support area.
Abbott - A Leader in Rapid Point-of-Care Diagnostics.