Global point of care
Maximising Operating Room Efficiency And Patient Safety With Point-Of-Care Testing
The Acute Care facility at King's College Hospital in the UK is one of the leading centers in Europe for clinical research, carrying out approximately 1,000 cardiothoracic operations each year within its cardiovascular operating rooms (CVORs).
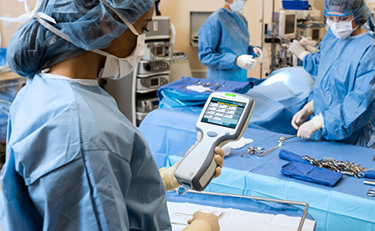
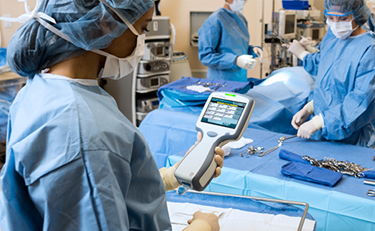
Throughout the hospital, point-of-care diagnostics are used within the cardiac operating theatres, the clinical perfusion team are supported by point-of-care testing for running blood gases in the form of the i-STAT Alinity instrument.
Through access to the handheld blood analyzers in the operating theatres, the team has been able to restructure their working patterns when treating patients, utilizing cardiopulmonary bypass for coronary artery disease, aortic and mitral valve stenosis or regurgitation, percutaneous aortic valve surgery and endocarditis of heart valves. With the use of the i-STAT Alinity the whole team has become more efficient which has increased their provision of quality care.