Global point of care
Point of Care Highlights
Featured Products
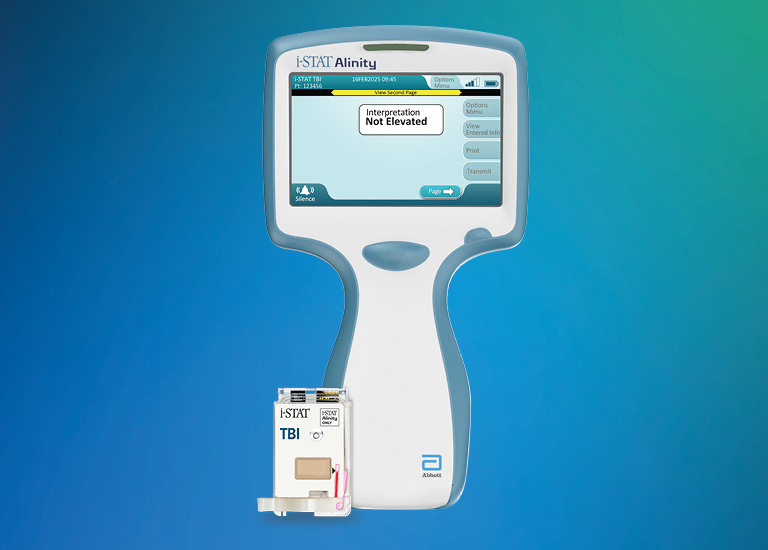
i-STAT CHEM8+ Cartridge
With i-STAT CHEM8+, healthcare professionals can obtain chemistries, electrolytes and hematocrit and hemoglobin levels in two minutes.
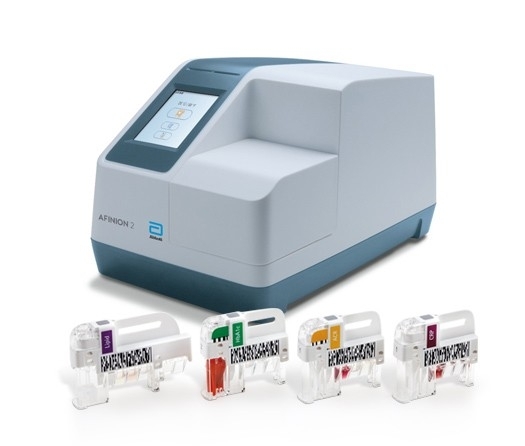
Afinion™ 2
The Afinion™ 2 analyzer is a compact, rapid, multi-assay analyzer that provides valuable near patient testing at the point-of-care. With the Afinion™ System, there’s no need to send test results to the lab or spend time tracking them down.
ID NOW™ PLATFORM
ID NOW™ Platform is a rapid, instrument-based, isothermal system for the qualitative detection of infectious diseases.
Service. Support. Answers.
Get all the detailed technical support and service you need – the way you need it. We’re here for you online and over the phone.


Service. Support. Answers.
Get all the detailed technical support and service you need – the way you need it. We’re here for you online and over the phone.