Play the Demo
Learn how the Digival™ Reader works by playing our interactive product demonstration.
BinaxNOW™ Influenza A & B Card 2 is an in vitro immunochromatographic assay for the qualitative detection of influenza A and B nucleoprotein antigens in nasopharyngeal (NP) swab and nasal swab samples. It is intended to aid in the rapid differential diagnosis of influenza A and B viral infections. BinaxNOW™ Influenza A & B Card 2 must be read by the the Digival™ Reader.
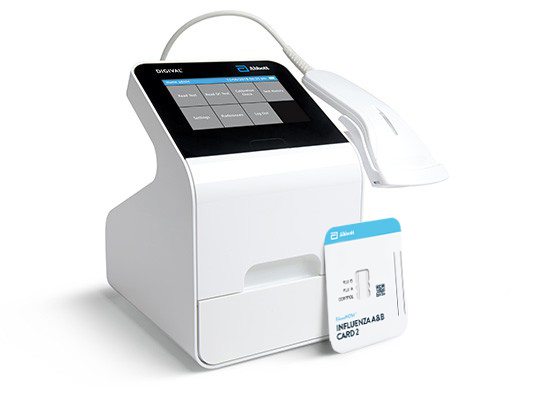
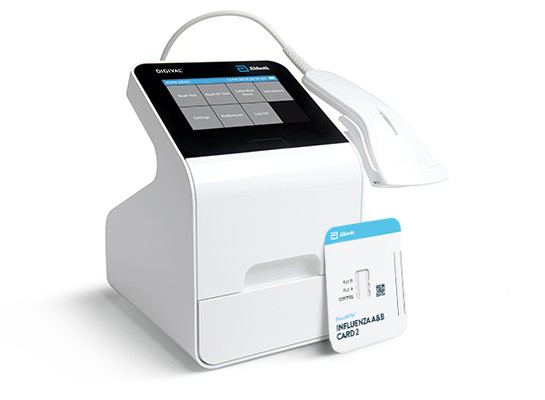

A Leader In Rapid Point-of-care Diagnostics.
©2026 Abbott. All rights reserved. Unless otherwise specified, all product and service names appearing in this Internet site are trademarks owned by or licensed to Abbott, its subsidiaries or affiliates. No use of any Abbott trademark, trade name, or trade dress in this site may be made without the prior written authorization of Abbott, except to identify the product or services of the company.
This website is governed by applicable U.S. laws and governmental regulations. The products and information contained herewith may not be accessible in all countries, and Abbott takes no responsibility for such information which may not comply with local country legal process, regulation, registration and usage.
Your use of this website and the information contained herein is subject to our Website Terms and Conditions and Privacy Policy. Photos displayed are for illustrative purposes only. Any person depicted in such photographs is a model. GDPR Statement.
Not all products are available in all regions. Check with your local representative for availability in specific markets. For in vitro diagnostic use only. For i-STAT test cartridge information and intended use, refer to individual product pages or the cartridge information (CTI/IFU) in the i-STAT Support area.
Abbott - A Leader in Rapid Point-of-Care Diagnostics.