Global Point of Care
A global challenge
Seasonal influenza, commonly referred to as “flu” or “flu season”, is often unpredictable and varies in severity.
It is one of the most common infectious diseases and is easily spread from person to person. The influenza virus attacks an estimated 5-10% of adults and 20-30% of children globally and causes substantial levels of illness, hospitalization, and death.1
Even though the most effective way to prevent influenza disease outbreak is vaccination, outbreaks of seasonal influenza still occur. Because the symptoms of influenza overlap with those of many other viral upper respiratory tract infections, people are often treated without a formal diagnosis.
That’s why we developed the unique isothermal nucleic acid amplification technology found in the ID NOW™ (formerly Alere™ i) platform. The rapid, instrument-based, isothermal system for the qualitative detection of influenza provides molecular results within 15 minutes.
3 to 5 million cases worldwide of severe illness annually1
250,000 to 500,000 deaths annually1
Influenza-associated annual costs in US:
$10.4 billion annually in direct medical costs and $16.3 billion indirect costs2


Making a positive difference in people’s lives
The development of the ID NOW technology was very exciting, not only from the perspective of seeing a concept become a commercial product but more importantly because it can truly provide a positive difference in people’s lives. Rapid Molecular point-of-care testing can provide actionable information in a very rapid timeframe, which is invaluable to the patient, the doctor, and the community.
- Rich Roth, CEO, Ionian Technologies (an Abbott company)*
Influenza can get anyone sick
Influenza symptoms begin abruptly, and are usually full blown within a few days of exposure, and include fever, sore throat, severe headache and overall body aches. For some people at high risk, influenza can cause severe illness or complications.4
George, a 68 year-old retired accountant, has been feeling unwell, with a sore throat and headache.



George’s symptoms
- High fever
- Dry cough and sore throat
- Body aches and headache
- Body chills
- Feeling tired and generally unwell
ACCURACY MATTERS
If you know now, you can act now. Taking antivirals within the first 48 hours of first symptoms helped shorten the length of the flu by 30% (1.3 days).6, 7
If you know now, you can act now. Taking antivirals within the first 48 hours of first symptoms helped shorten the length of the flu by 30% (1.3 days).6, 7
Most people who get the flu treat themselves at home and often don't need to see a doctor. But, for those with complications, seeing a doctor right away and antiviral drugs within the first 48 hours after first symptoms appear may reduce the length of illness and help prevent more-serious problems.4
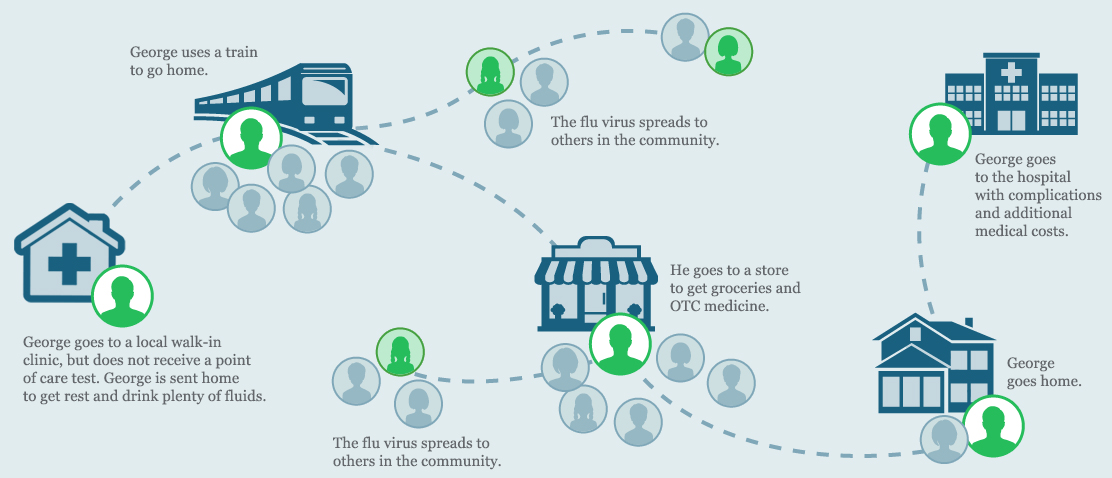
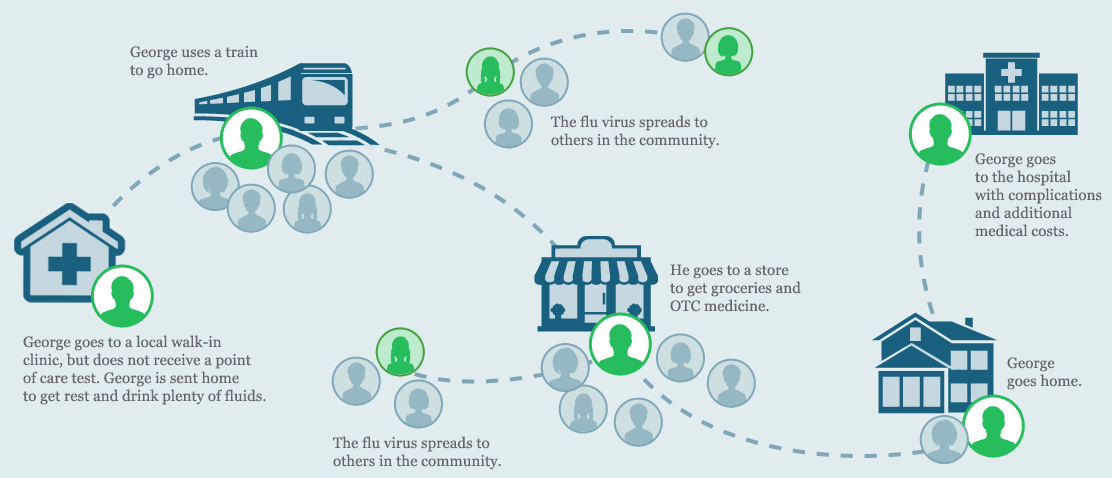
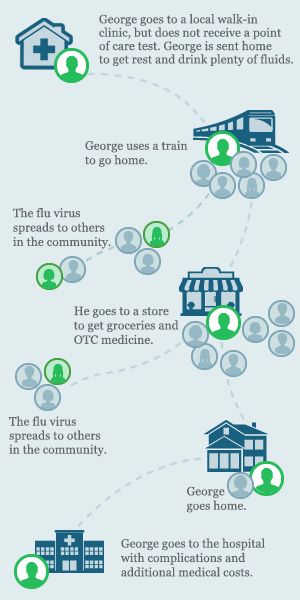
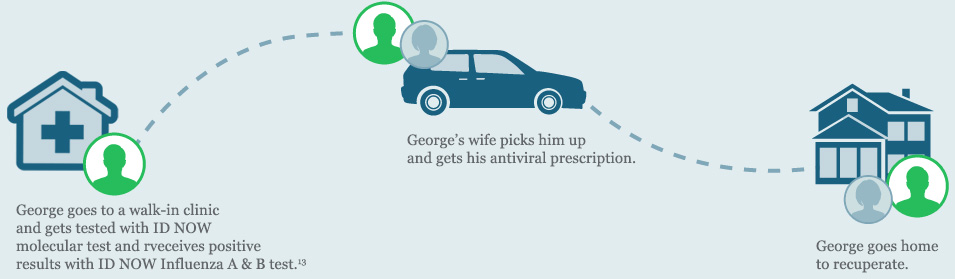
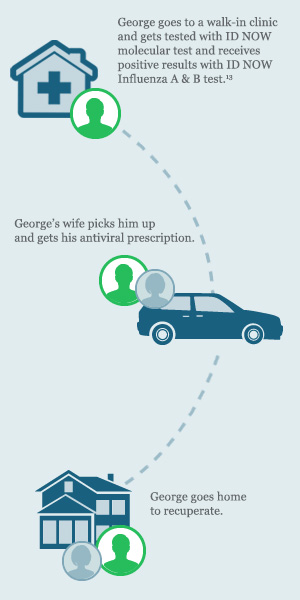
George goes to a local walk-in clinic after he starts noticing his symptoms. The doctor examines him, but does not order an influenza point of care test. George is sent home with instructions to get rest and drink plenty of fluids.
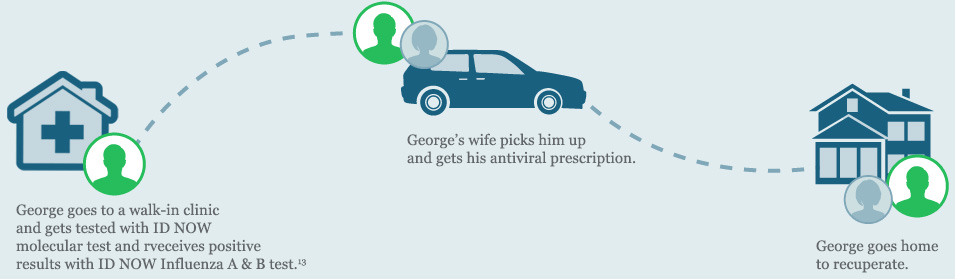
After a few days, George’s symptoms get worse and he has trouble breathing. His wife takes him to the hospital and his doctor orders a CLIA-waived ID NOW Influenza A B molecular test.
The challenge of diagnosing influenza
Since influenza is highly contagious, the last thing you want is for infectious people to linger in waiting rooms or out in the community, risking further infection of patients, medical staff, and others in the community.1
Confidence in the molecular result
Rapid diagnosis of influenza can lead to reduced hospital stays, reduced secondary complications, and reduced cost of hospital care.10, 11
Although conventional rapid influenza tests have a fast turnaround time and good specificity, the sensitivity is lacking.8
Rapid Molecular diagnostics, like the ID NOW system, bridge the gap between accuracy and speed. ID NOW is significantly faster than other molecular methods and more accurate than conventional rapid tests.
Molecular sensitivity in an actionable timeframe allows the prudent use of antibiotics and antivirals. Antibiotics are not effective against influenza, and early diagnosis of influenza can reduce the inappropriate use of antibiotics.9
Rapid diagnosis of influenza can lead to reduced hospital stays, reduced secondary complications, and reduced cost of hospital care.10, 11
Confidence in the molecular result
Although conventional rapid influenza tests have a fast turnaround time and good specificity, the sensitivity is lacking.8
Rapid Molecular diagnostics, like the ID NOW system, bridge the gap between accuracy and speed. ID NOW is significantly faster than other molecular methods and more accurate than conventional rapid tests.
Molecular sensitivity in an actionable timeframe allows the prudent use of antibiotics and antivirals. Antibiotics are not effective against influenza, and early diagnosis of influenza can reduce the inappropriate use of antibiotics.9
Timely and accurate diagnosis is essential for appropriate influenza treatment12
Rapid diagnostic tests with increased sensitivity are essential for the reliable detection of influenza A and B and enable immediate, effective treatment decisions.5, 10, 11
The potential to change lives is a driving force behind what we do.
Part of the solution
Rapid and accurate diagnosis of viral infection is critical to reducing the disease burden of influenza and its social and economic consequences. Since taking antiviral drugs within the first 48 hours after first symptoms appear may reduce the length of illness and help prevent more-serious problems with influenza, it is important to get accurate test results early.
Rapid influenza diagnosis can help doctors make the right treatment decisions and improve patient outcomes, fast.
Part of the solution
The potential to change lives is a driving force behind what we do.
Rapid and accurate diagnosis of viral infection is critical to reducing the disease burden of influenza and its social and economic consequences. Since taking antiviral drugs within the first 48 hours after first symptoms appear may reduce the length of illness and help prevent more-serious problems with influenza, it is important to get accurate test results early.
Rapid influenza diagnosis can help doctors make the right treatment decisions and improve patient outcomes, fast.
View References
*Ionian Technologies, LLC is a wholly owned subsidiary of Alere, Inc.
1. World Health Organization (WHO). Influenza Fact Sheet. March 2014. http://www.who.int/mediacentre/factsheets/fs211/en/
2. Molinari N-AM. et al. The annual impact of seasonal influenza in the US: measuring disease burden and costs. Vaccine. 2007;25:5086–96.
3. CDC Foundation. Flu Prevention and Business Challenges Infographic. http://www.cdcfoundation.org/businesspulse/flu-prevention-infographic. Page accessed October 13, 2015.
4. Centers for Disease Control and Prevention (CDC). What you should know for the 2015-2016 influenza season. http://www.cdc.gov/flu/about/season/flu-season-2015-2016.htm. Page updated August 25, 2015.
5. Centers for Disease Control and Prevention (CDC). Guidance for Clinicians on the Use of Rapid Influenza Diagnostic Tests. http://www.cdc.gov/flu/professionals/diagnosis/clinician_guidance_ridt.htm. Page updated: November 13, 2014.
6. Tamiflu® (oseltamivir phosphate) Prescribing Information. South San Francisco, CA: Genentech USA, Inc.; January, 2013.
7. Rapivab [package insert]. Durham, NC: Biocryst Pharmaceuticals, Inc; 2014.
8. Centers for Disease Control and Prevention (CDC). Rapid Diagnostic Testing for Influenza: Information for Clinical Laboratory Directors. http://www.cdc.gov/flu/professionals/diagnosis/rapidlab.htm. Page updated November 5, 2014
9. Centers for Disease Control and Prevention (CDC). Clinical Description & Lab Diagnosis of Influenza. http://www.cdc.gov/flu/professionals/diagnosis/index.htm. Page updated October 16, 2014.
10. Bonner, A.B. et al. (2003) Impact of the Rapid Diagnosis of Influenza on Physician Decision-Making and Patient Management in the Pediatric Emergency Department: Results of a Randomized, Prospective, Controlled Trial. Pediatrics. Vol. 112 No. 2.
11. Blaschke AJ, Shapiro DJ, Pavia AT, Byington CL, Ampofo K, Stockmann C, Hersh AL. A National Study of the Impact of Rapid Influenza Testing on Clinical Care in the Emergency Department. J Pediatric Infect Dis Soc. 2014 Jun;3(2):112-118. Epub 2013 Nov 13.
12. Centers for Disease Control and Prevention (CDC). Influenza Symptoms and the Role of Laboratory Diagnostics http://www.cdc.gov/flu/professionals/diagnosis/labrolesprocedures.htm. Page updated: October 16, 2014.
13. Alere™ i Influenza A & B Product Insert.



Featured in this story



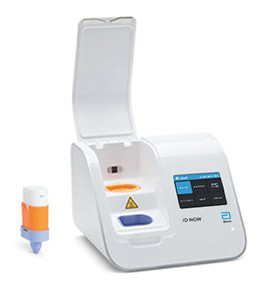
ID NOW™ Platform
Significantly faster than other molecular methods and more accurate than conventional rapid testing, giving you the confidence to make effective patient management decisions sooner.
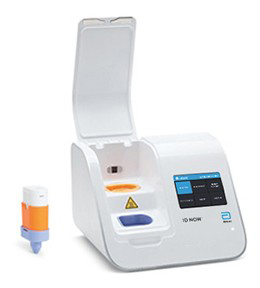

ID NOW™ Influenza A & B 2 test
The ID NOW™ Influenza A & B 2 is the first CLIA-waived molecular rapid flu test delivering molecular flu results in less than 15 minutes on our unique ID NOW™ platform.



