Global Point of Care
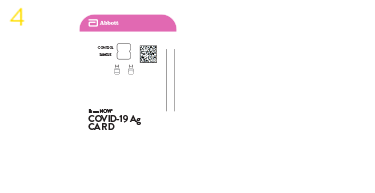
BINAXNOW™ COVID-19 Ag card
FAST, EASY AND COST-EFFECTIVE
COVID-19 TESTING
When it comes to testing for COVID-19, your patients are looking for answers — and you're looking for a fast, easy and cost-effective way to help. Abbott has designed the BinaxNOW™ COVID-19 Ag Card for decentralized testing, so you can test right at the point of care and get results for your patients quickly and easily.
The BinaxNOW COVID-19 Ag Card test has received U.S. Food and Drug Administration (FDA) Emergency Use Authorization (EUA).*
Only available in the US.



References
*The BinaxNOW COVID-19 Ag Card has not been FDA cleared or approved. It has been authorized by the FDA under an emergency use authorization. It has been authorized only for the detection of proteins from SARS-CoV-2, not for any other viruses or pathogens, and is only authorized for the duration of the declaration that circumstances exist justifying the authorization of emergency use of in vitro diagnostics for detection and/or diagnosis of COVID-19 under Section 564(b)(1) of the Federal Food, Drug and Cosmetic Act, 21 U.S.C. § 360bbb-3(b)(1), unless the declaration is terminated or authorization is revoked sooner.
** Before testing patients, federal regulations require testing sites to have a CLIA certificate issued by CMS. Sites performing only waived tests must obtain a Certificate of Waiver by applying for this certification for each location performing testing.
***Refer to the product package insert for full instructions, clinical data and information on serial testing.
- FDA Letter "Revisions Related to Serial (Repeat) Testing for the EUAs of Antigen IVDs." November 1, 2022. Accessed March 3, 2023. https://www.fda.gov/media/162799/download