Global Point of Care
DIGIVAL™ Installation Guide
Just received your DIGIVAL™?
Follow the 4 easy steps: Installation, Run Calibration Check, Read QC Test, and Read Patient Sample to begin setting up your DIGIVAL. Click on each step below to reveal the instructions and get started.
When you have successfully installed your DIGIVAL, please let us know by clicking the button in the ‘Installation Complete’ section at the end of this guide.



Step 1: Installation
In Step 1 you will unpack and check all contents for damage and familiarize yourself with DIGIVAL. Then set it up and log in for the first time.
Unpack and Check Contents
Unpack you equipment and check contents for damage.
Check to make sure that the following components are included in the packaging:
- DIGIVAL and power cord/adapter
- Barcode Scanner Bracket
- DIGIVAL User Manual (and Multi-language CD in select markets)
- DIGIVAL Quick Start Guide
- Abbott USB Drive
A separate package contains:
- Barcode Scanner
- Barcode Scanner Holster and thumbscrew
- Test Insert Tray Pack (assay specific)
- Calibration Check Card Pack
Contents
LFR-000
LFR-024



DIGIVAL



Barcode scanner bracket
INLFR000
INLFR024



DIGIVAL User Manual
LFR-004



BinaxNOW™ Insert Tray Pack
OPR2001ZWU1-201
L22XWU1-200



Barcode scanner and holster



Power Supply
LFR-003



Calibration Check Card Pack
Optional Accessories
MCP181035



Printer
EQ004003



Abbott USB Memory Stick
DIGIVAL At A Glance
DIGIVAL, Front View
- Power On/Off Button
- Test Insert Tray



DIGIVAL, Back View
- Barcode scanner bracket location holes
- USB Port (x1)
- Power connection
- Ethernet
- USB Port (x2)



Set Up
- Place DIGIVAL on the bench with access to power supply.



- Assemble the barcode scanner
- Connect the bracket assembly to the back of DIGIVAL



- Attach the barcode scanner to USB and place into the holder



- Connect the power supply and turn on DIGIVAL






First time Log In
When logging in for the first time you will need to:
a) Log In as Admin
b) Set the date
c) Set the time
d) Set the admin password
e) Create operator(s)
f) Set Walk Away or Read Now mode
g) Log out
a) Power up and Log in as “admin”.
- The first user to power up the system can log in as “admin.”
- To log in:
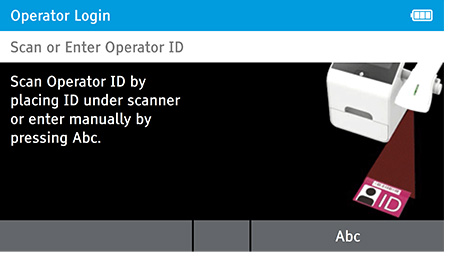
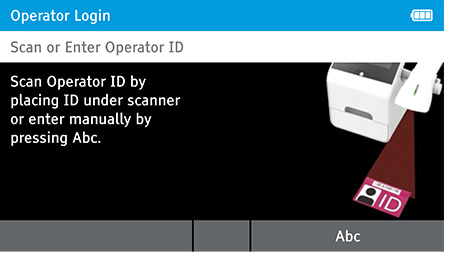
- Scan or Enter Operator ID: Enter 'Admin' as the login name.
- Password: Enter 'admin' for the password.


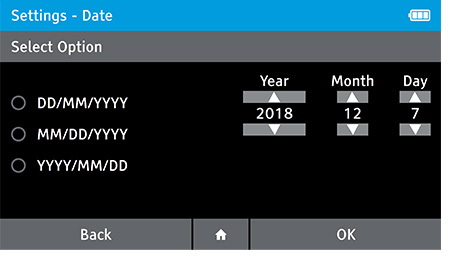
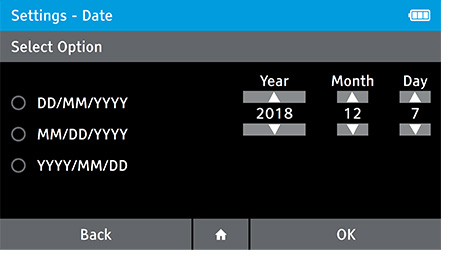
b) Set the date.
- To set the date tap: Settings>Date and Time>Date
- Select the preferred date format. Set the correct date using the up and down arrow keys on the touch screen to move between day, month and year.
- Press OK to advance.

c) Set the time.
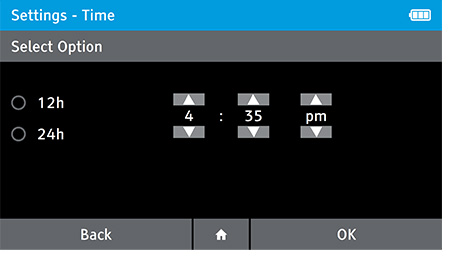
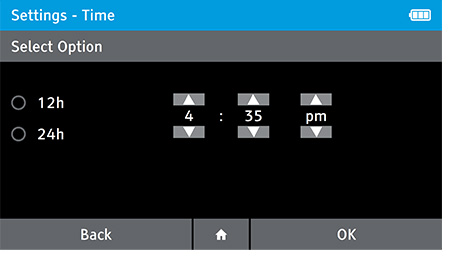
- This screen will be displayed automatically once the date is set.
- Set the correct time using the up and down arrow keys, and choose between a 12 Hour or 24 Hour display using the touch screen.
- Press OK to advance.

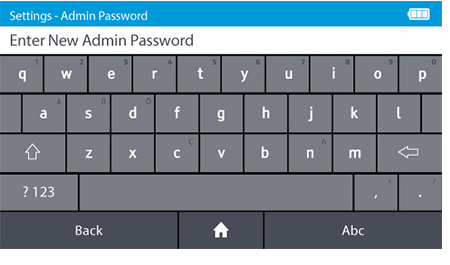
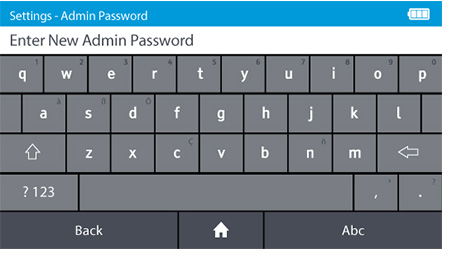
d) Set the new Admin password.
- DIGIVAL is supplied with a prefixed admin ID. The default admin password is "admin".
- The Admin password can be changed. To change the admin password tap: Settings>Access control>Admin Password
- Passwords are case-sensitive and must be alphanumeric. The password cannot contain spaces. Passwords must be 4-20 characters.
Note: If the Admin password is forgotten the Admin must contact Abbott™ Technical Support.

e) Create operators
- To create operator login permissions, tap: Settings>Access Control>Operators
- Tap Actions.
- Tap Add. The keypad displays, with the message, “Scan Operator ID or enter manually.”
- Enter the Operator ID (for example the Operator's name) or scan Operator's ID using the electronic barcode, then tap OK.
- The Operator ID must be 2-20 characters, and cannot contain spaces or special characters.
- Operator ID's are not case sensitive. Use the 123 or Abc keys to switch between the alphanumeric and numeric keypads.
- Add at least one operator so that you can perform a Calibration Check.
- You can return to this screen to add the rest of the staff at any time.

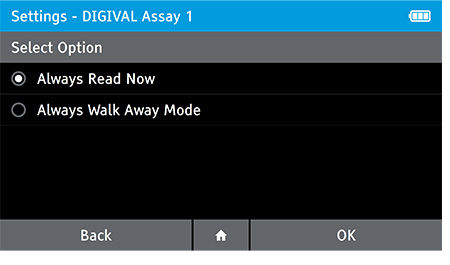
f) Set Walk Away or Read Now mode
- Use the Walk Away Mode menu option to configure patient tests to be run in Walk Away Mode or Read Now Mode. Read mode can be configured for each assay on the DIGIVAL.
- Always Read Now: After the incubation time indicated in the Package Inserts, the DIGIVAL will read a test immediately after inserting device.
- Always Walk Away Mode: DIGIVAL will always time assay development and interpret the test result at the time indicated in the package insert.
- To set the read mode tap: Home>Settings>Test Setting>Walk Away Mode
- Select the Assay which you wish to set the read mode for. Next, select the read mode and tap OK.

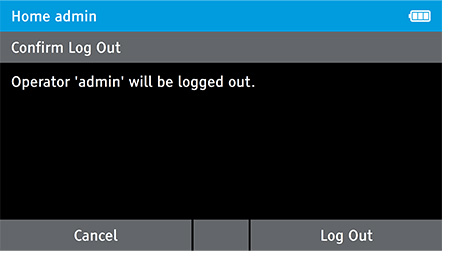
g) Log Out
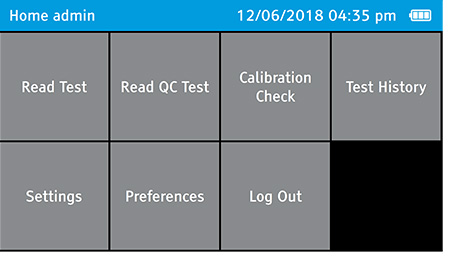
- Log out as the admin.
- To log out, tap: Home>Log Out


Step 2: Run Calibration Check
A Calibration Check must be performed before the first test can be processed and then at least every 30 days after initial calibration check.
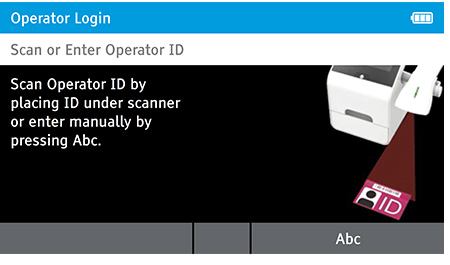
a) Login
- Enter the Operator ID using the barcode scanner or enter manually using the keyboard by pressing Abc.
- If a password is required (as specified by the Admin during setup) a prompt will appear to enter the password.

b) Select Calibration Check on the home screen
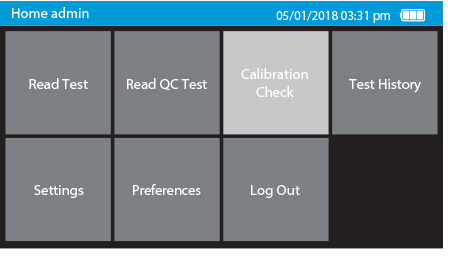

c) Remove Tray
- When the user selects “Calibration Check” on the home screen the device will self-check to ensure there is adequate memory.
- Next, the user will be prompted to remove the tray from inside the drawer in order to place the Calibration Check Card in.
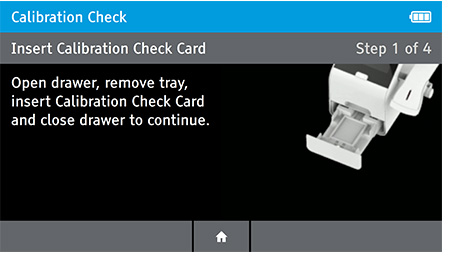

Remove Insert Tray
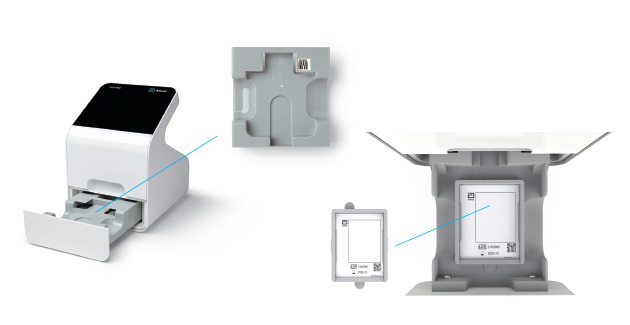
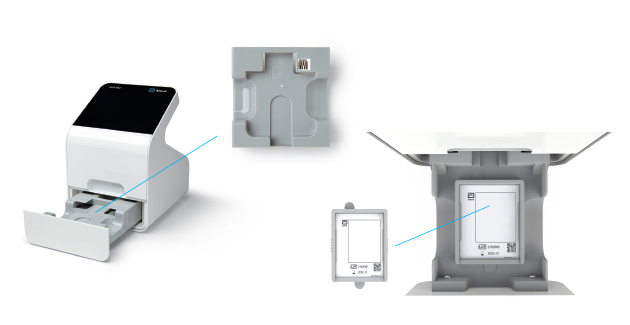

Insert Calibration Check Card
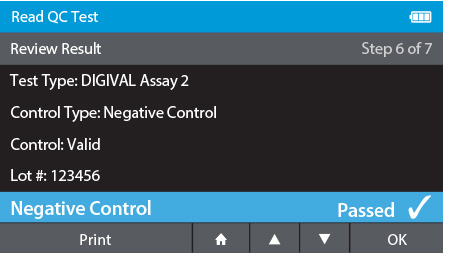
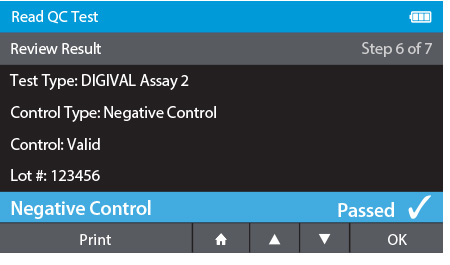
d) Review Result
- When complete the result will be displayed for review.
- The user has the option to print the result or press the drawer in order to place the Calibration Check Card.
- If user has the Home button selected the user will be prompted to open the drawer, replace the tray and close the drawer.
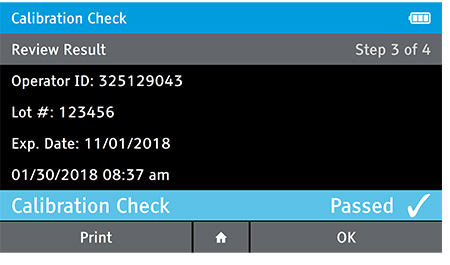
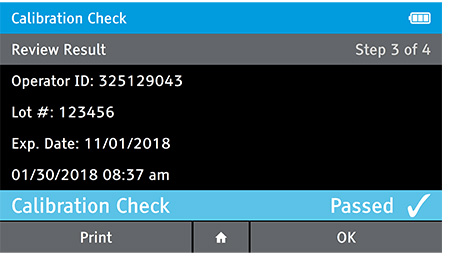

e) Replace Tray
- The user will be prompted to open the drawer, remove the Calibration Check Card, replace the tray and close the drawer.
- Ensure you return the calibration card carefully back into the light block bag. When drawer is closed the Home screen will appear.
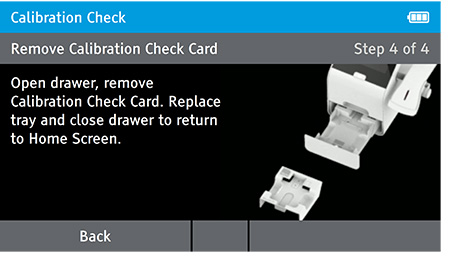
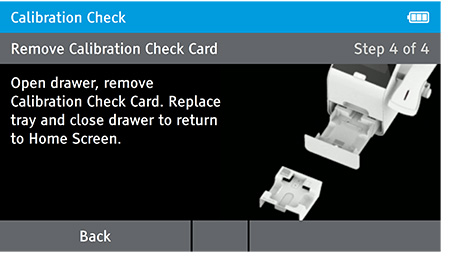

NOTE: Return the calibration check card to the light-block bag in which in it was delivered. The calibration check card must always be stored in the light block bag when not in use.
Step 3: Read a QC Test
BinaxNOW™ Influenza A & B Card 2 kits contain Influenza A & B Positive and Viral Negative Control Swabs. The Influenza A and B Positive Control swab should generate a positive result for both Influenza A and B when read by DIGIVAL the positive result will be displayed as Passed. These swabs will monitor the entire assay. Test these swabs with each new shipment received and once for each untrained operator. Other controls may be tested in order to conform with: local, state and/or federal regulations, accrediting groups, and/or, your lab’s standard Quality Control procedures.
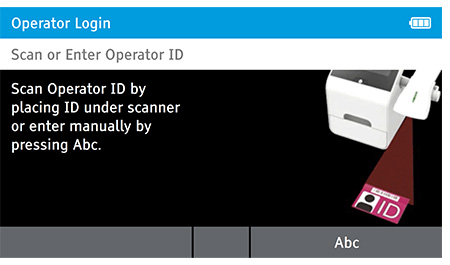
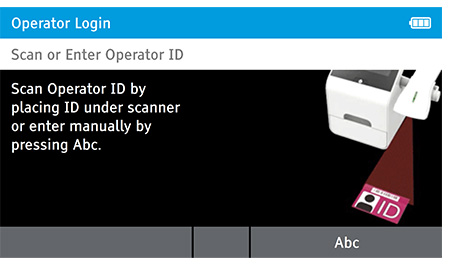
When DIGIVAL is switched on, or after another Operator has logged out, the Scan or Enter Operator ID screen will be displayed. Enter the Operator ID using the barcode scanner or enter manually using the keyboard by pressing Abc. If a password is required (as specified by the Admin during setup) a prompt will appear to enter the password.
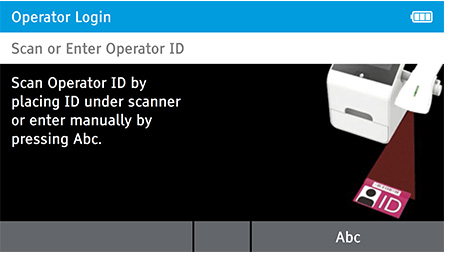
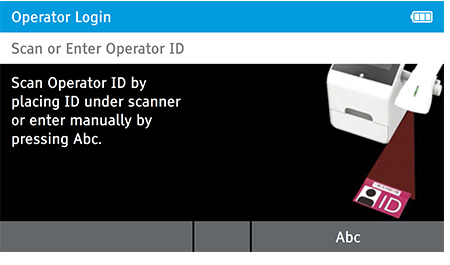

To Read QC test tap home > Read QC test

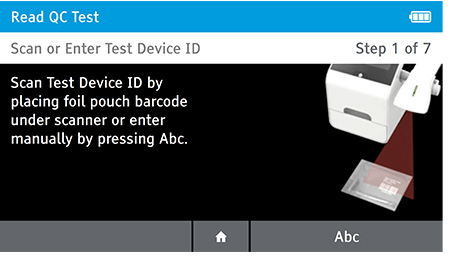
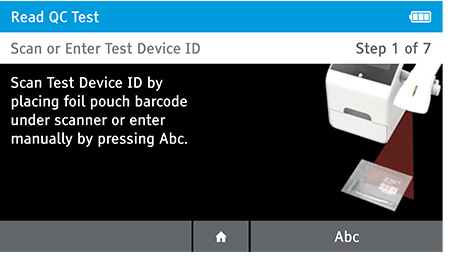
a) Scan or enter test device ID.

b) Select QC test to be run.
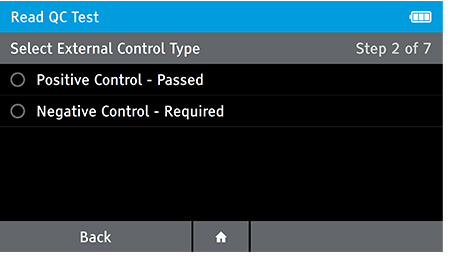

c) Refer to Package Insert for QC preparation and test procedure.
d) Enter QC standard ID, if configured. Home > Test Settings > QC Standard ID.
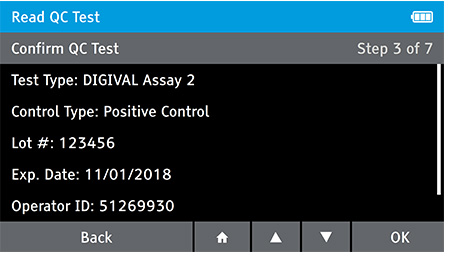

e) Insert test at appropriate time depending on Read Now or Walk away mode.
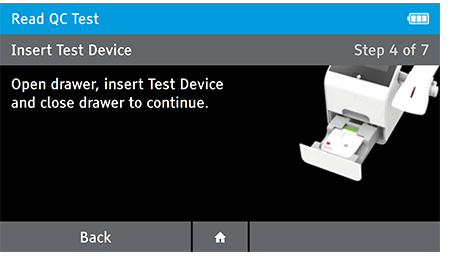

Read Now mode - At the read time indicated in assay instructions, open drawer, insert test device and close drawer.
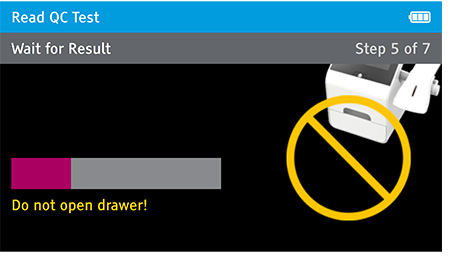

Walk away mode - Once procedure is complete and the test device is securely closed, immediately open drawer, insert test device and close drawer. Reader will time the test completion for the user.
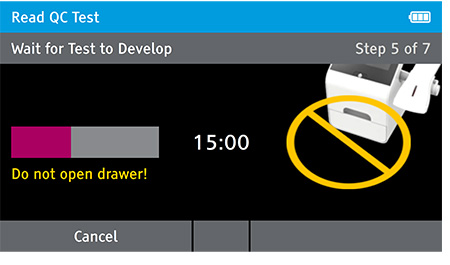

f) When test completes successfully remove test card.
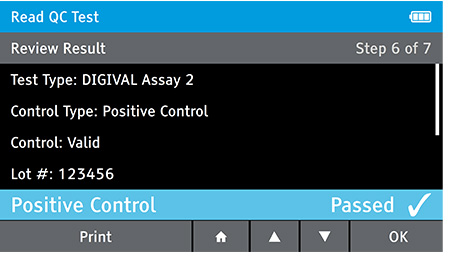


g) Repeat Steps for each QC control needed.
NOTE:If the QC controls do not perform as expected, repeat the external control testing process. You may also contact your Supervisor or DIGIVAL Technical Support at 1.888.735.5317 for assistance before testing patient specimens. |
Step 4: Read a Patient Sample
When DIGIVAL is switched on, or after another Operator has logged out, the Scan or Enter Operator ID screen will be displayed. Enter the Operator ID using the barcode scanner or enter manually using the keyboard by pressing Abc. If a password is required (as specified by the Admin during setup) a prompt will appear to enter the password.

1. Tap Read Test

a) Scan or enter the test device ID.
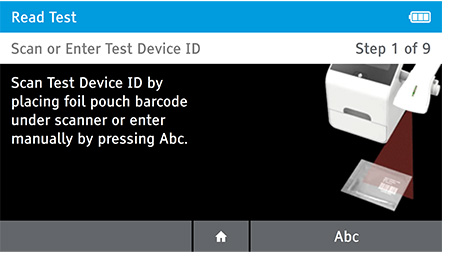
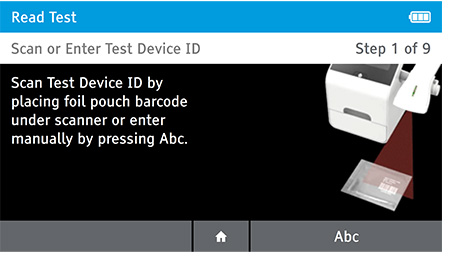

b) Scan or enter patient ID.
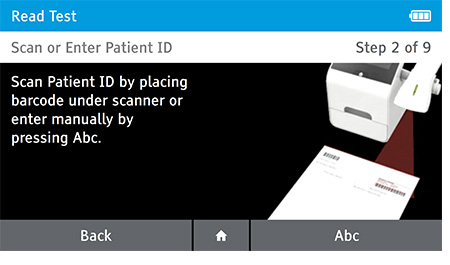
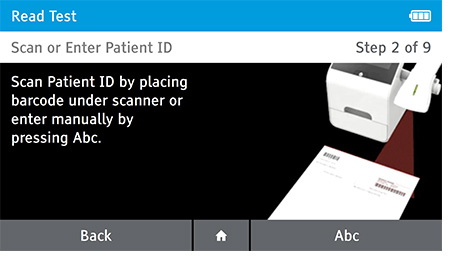

c) Confirm Patient and Test information. Patient information and Test Type cannot be edited once testing has begun.
d) Refer to the assay Package Insert for sample preparation and assay procedure.
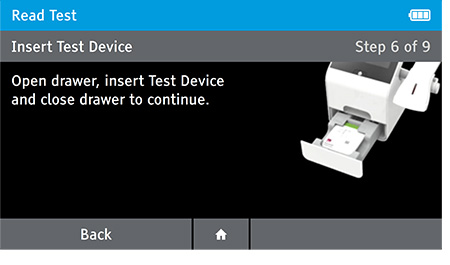
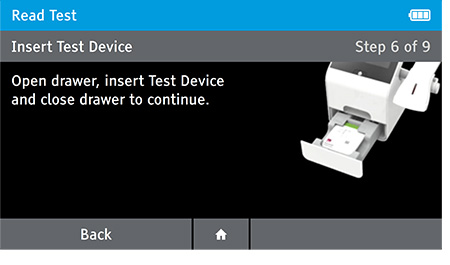

e) Insert test device at the appropriate time depending on read mode.
Read Now mode - At the read time indicated in assay instructions, open drawer insert test device and close drawer.
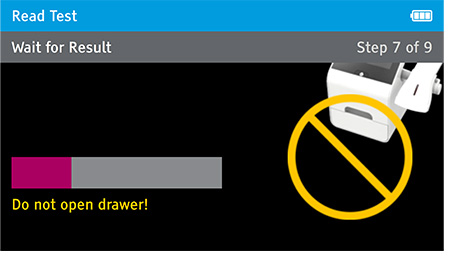

Walk away mode - Once procedure is complete and the test device is securely closed, immediately open drawer, insert test device and close drawer. Digival will time the test completion for the user and read the result at the read time.
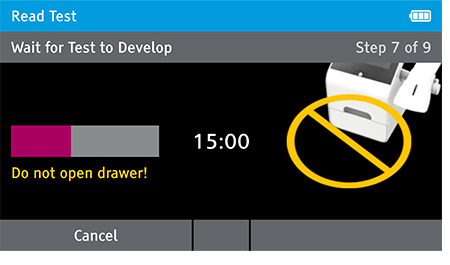

f) When test completes successfully, results are displayed on the screen with positive or negative results.
g) If optional printer is setup tap print to print the result.
h) Open the drawer and discard the test device.
Maintenance
DIGIVAL is maintenance-free and has no serviceable parts. In case of Digival failure or damage, contact Abbott Technical Support 1.888.735.5317
Cleaning the DIGIVAL surfaces and test device tray:
Clean the exterior DIGIVAL surfaces and the test device tray daily, and immediately after possible patient sample contamination. Use a lint free cloth and a 70% ethanol, 70% isopropanol or 10% bleach solution. Do not spray or pour solution directly onto the DIGIVAL when cleaning. Ensure no excess liquid is used when cleaning as it may damage the DIGIVAL.
Clean surrounding bench area. Clean DIGIVAL and surrounding area immediately after possible patient sample contamination.
Technical Services
If you need additional assistance you may request a call from technical services or call directly.
For immediate help, call technical services at 1.888.735.5317.
Installation Complete
When you have successfully completed the online installation program, please let us know by clicking the button below.
Helpful Documents
To view the package insert and other helpful documents, click here.
Videos
Operating DIGIVAL™ (US)





