Global point of care
ABBOTT POINT OF CARE, INC.
400 COLLEGE ROAD EAST
PRINCETON, NJ 08540
T: +1 609 454 9000
F: +1 609 419 9370
December 2020
Urgent Medical Device Correction
|
PRODUCT NAME |
LIST NUMBER |
PRODUCT NAME |
LIST NUMBER |
|---|---|---|---|
|
i-STAT CG8+ cartridges |
03P88-25 |
i-STAT EG7+ cartridge |
03P76-25 |
Dear Valued Abbott Point of Care Customer,
This letter contains important information regarding the i-STAT CG8+ and EG7+ cartridges noted above. If you are using these cartridges, your facility may be impacted, and further review and action may be required.
Abbott Point of Care (APOC) is communicating this information to all potentially impacted customers. Please review the information below as your attention to this information is required. Note: information in this letter is lot number specific.
PRODUCT DESCRIPTION
The i-STAT CG8+ cartridge contains eight measured tests (sodium, potassium, ionized calcium, pH, pCO2, pO2, glucose and hematocrit) and the i-STAT EG7+ cartridge contains seven measured tests (sodium, potassium, ionized calcium, pH, pCO2, pO2, and hematocrit).
The test for ionized calcium, as part of the i-STAT System, is intended for use in the in vitro quantification of ionized calcium in arterial, venous, or capillary whole blood.
Description of the issue
The i-STAT CG8+ and EG7+ cartridge Instructions for Use (IFU) indicate that these cartridges can be stored for 2 months at room temperature (18 to 30° C). Abbott routinely tests performance of i-STAT cartridges, including stability testing of the i-STAT CG8+ and EG7+ cartridges at various time intervals after storage in the refrigerator and at 30°C.
Through this internal testing process after storage at 30°C for 14 days, Abbott Point of Care observed approximately 5.2% of cartridges reporting higher than expected reported results for ionized calcium. Abbott Point of Care observed ionized calcium on some i-STAT CG8+ or EG7+ cartridges may exhibit higher than expected reported results when stored at the room temperature (18 to 30° C) for periods of time in excess of:
- 3 days for lot numbers *20100 to 20339
- 7 days for lot numbers *20340 and above
Where * = lot letter A, K, L, M, N, W, or Y
Abbott Point of Care has not observed higher than expected reported results for ionized calcium test when i-STAT CG8+ and EG7+ cartridges are removed from refrigerated storage and warmed to room temperature for use within the number of days indicated above.
Abbott Point of Care has not observed this issue for any tests on the i-STAT CG8+ or EG7+ other than the ionized calcium test.
Abbott Point of Care is communicating this information to potentially impacted customers. Clinicians should be advised to consider a patient's signs, symptoms, history, and results of other diagnostic tests when interpreting the ionized calcium results from these cartridges. If the results do not match the patient's clinical presentation, the patient sample should be retested using an alternate i-STAT cartridge or test method. Treatment of falsely high ionized calcium may result in harm, by precipitating hypocalcemia. Abbott Point of Care has determined that the overall likelihood of harm associated with this issue is rare. The most common symptoms of hypocalcemia include paresthesia, muscle spasms, cramps, tetany, circumoral numbness, and seizures. Although improbable, in the most extreme cases, hypocalcemia could potentially result in sudden cardiac death. Abbott Point of Care has not received any reports of patient harm associated with this issue.
RECOMMENDED ACTIONS
Our records show that your facility is an i-STAT CG8+ or EG7+ cartridge user. While Abbott Point of Care continues to investigate this issue, if reporting ionized calcium is required, it is recommended that the i-STAT CG8+ and EG7+ cartridges be used only:
- within 3 days of removal from refrigerated storage for lot numbers between *20100 and *20339
- within 7 days of removal from refrigerated storage for lot numbers *20340 and above
Where * = lot letter A, K, L, M, N, W, or Y
If your facility is unable to manage to the recommended action outlined above, Abbott Point of Care recommends that your facility disable and not report the ionized calcium test on the i-STAT CG8+ and EG7+ cartridges and the use an alternate i-STAT cartridge for reporting ionized calcium. Please see additional information below on how to disable the ionized calcium test for these cartridges.
Please confirm receipt and understanding of this communication by responding to the business reply form included with this letter.
If you have forwarded any i-STAT CG8 + and EG7+ cartridges to another facility, we request that you please provide a copy of this letter to them.
We are providing, as an attachment, a list of the potentially impacted i-STAT CG8+ and EG7+ cartridge lots that have been distributed as of the date of this letter. Please continue to follow the recommended actions in this communication for all newly received i-STAT CG8+ and EG7+ lots until your facility receives an updated communication from Abbott Point of Care.
ADDITIONAL INFORMATION
Your Abbott Point of Care sales and support team will be contacting you to review interim solutions for the challenges this communication may bring. If you have any questions regarding this information, please contact Abbott Point of Care Sales Support services representative. Additionally, please report any questions or concerns about the performance of the ionized calcium test on the i-STAT CG8+ and EG7+ cartridges to Abbott Point of Care Technical Support or via email at oustechsvc@apoc.abbott.com .
If your facility decides to proceed with disabling the ionized calcium test, instructions are available on the Abbott Point of Care website https://www.pointofcare.abbott. If ionized calcium is interfaced through the Laboratory Information System (LIS) interface, disabling the analyte may require a change to the interface. Please contact your interface vendor for support.
Abbott Point of Care is fully committed to addressing this situation as a top priority and supporting you throughout this process. Abbott Point of Care will provide timely follow up communications when there is data to support longer storage at room temperature and after completion of the investigation.
Resources for customization for the i-STAT Alinity instrument:
- Go to https://www.pointofcare.abbott and navigate to Support >
i-STAT Alinity Resources Login > Operator Documentation > i-STAT Alinity System Operations Manual
(Language: English): - Customers using AlinIQ CWi, refer to i-STAT Alinity System Operations Manual, Section 3.1 - AlinIQ CWi - Customization Workspace for i-STAT, Analyte Settings Category, Enable/Disable Analyte for information on customizing this option.
NOTE: To customize this feature AlinIQ Cwi is required.
Resources for customization for the i-STAT 1 Analyzer:
- Go to https://www.pointofcare.abbott and navigate to Support >
i-STAT 1 Resources Login > Administrator Documentation > Technical Bulletins (Language: English United Kingdom) >
Data Management User Documentation and select i-STAT DE Documenta - Refer to the i-STAT DE User Guide v2.10 Art:754472, Section 3.15 Customizing Analyte Enable Options
- Go to https://www.pointofcare.abbott and navigate to Support >
i-STAT 1 Resources Login > User Documentation > i-STAT 1 System Manuals - i-STAT Customers without i-STAT/DE or CDS, refer to the i-STAT System Manual, i-STAT 1 Analyzer 2 Art:714364, Changing the Profile for guidance on navigating to the Results menu, then the Units and Ranges menu to change the setting.
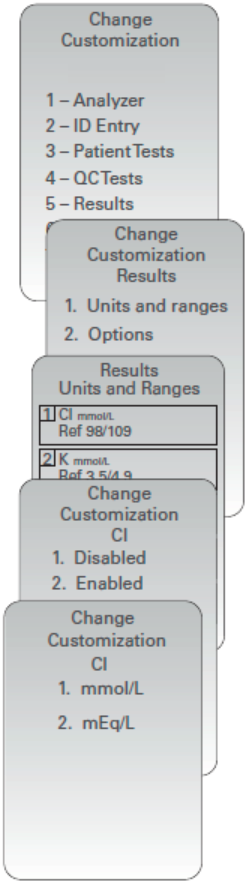
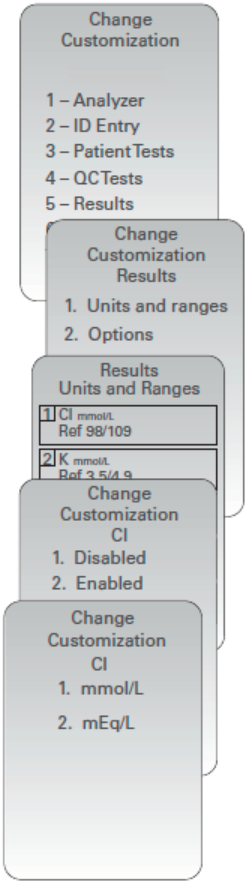
Customizing Analyte Enable Options using the i-STAT 1 Analyzer Keypad
New analyzers or replacement analyzers will have standard unit sets installed and all analytes enabled. To disable or set a different unit of measurement for a particular analyte globally, access Change Customization > Units and Ranges. Perform the following steps:
NOTE: This setting will apply globally to all cartridge types used. To disable analytes by i-STAT Cartridge panel, users must use i-STAT/DE or CDS.
- Press
 to turn on the analyzer.
to turn on the analyzer. - Press
 to change to the Administration Menu
to change to the Administration Menu - Press
 (Customization)
(Customization) - Press
 (Change)
(Change) - Press
 (when enabled, enter password)
(when enabled, enter password) - Press
 (Results)
(Results) - Press
 (Units and Ranges)
(Units and Ranges) - Press the number key corresponding to the analyte you
wish to disable or change units for.
Press or
or  arrow key to return to the analyte selection page.
arrow key to return to the analyte selection page. - Press 1- (Disabled), to disable the analyte OR
Press 2- (Enabled) to change the units. - When changing units, press the number key corresponding
to the units in which you would like the analyted reported. - Once all analytes are set as you wish to report, press twice
to save and return to the Main Menu.
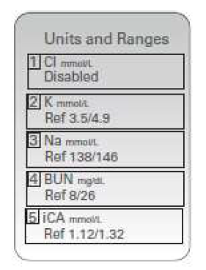
NOTE: When an analyte is disabled, units and ranges will not be displayed on the Results Units and Ranges screen.
APOC2020-007