Global point of care

Gene Deletion and Malaria: A Call to Action
Malaria remains a significant global health threat which has become increasingly complicated due to factors like PfHRP2/3 gene deletion that led to HRP-2 based rapid tests false negatives, ultimately missing sick patients.1,2 Rapid tests that detect the malaria parasite that do not express the HRP protein may be able to help improve diagnosis.
Why it Matters
PfHRP2/3 gene deletion compromises the accuracy of diagnosis, increasing the risk of false-negative test results and hindering effective disease control and surveillance efforts.1,2
The good news? We’re working on next-generation malaria rapid diagnostic tests (RDTs) that can help overcome PfHRP2/3 gene deletion issues and help create a healthier community.
Understanding Gene Deletion
Gene deletion is a genetic phenomenon in which a segment of DNA that encodes a specific gene is entirely missing or absent from an individual's genetic code. Gene deletions can occur naturally as a result of mutations during DNA replication or recombination, or they can be inherited. Depending on the gene affected and the role it plays in the parasite, gene deletions can have a wide range of consequences, such as causing genetic disorders, influencing susceptibility or resistance to certain infectious diseases like malaria, or impacting accuracy of diagnostic tests.3
The Malaria Connection
HRP2-negative parasites present one of the most pressing global threats to the fight against malaria as they increase the risk of false negative test results.1 This is largely because HRP2-negative parasites cannot be detected by conventional malaria Pf RDTs, which target HRP2 only.2 Because many of the geographic locations impacted by these parasites rely on conventional RDT technology to effectively diagnose and guide treatment, many people could be misdiagnosed.5
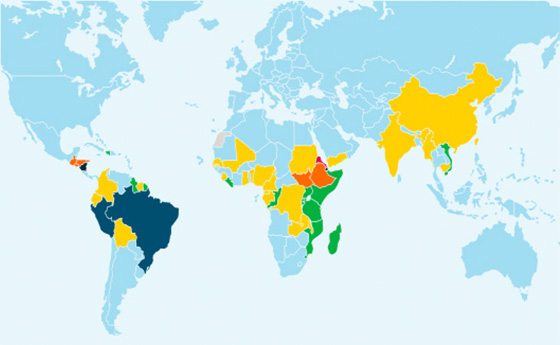
Estimated prevalence of Pfhrp2 gene deletions 2022

Significant prevalence of HRP2-negative parasites has been shown in many countries. However, surveillance data is limited, making it difficult to raise awareness and help people understand the appropriate magnitude of this issue.4
How is Malaria Diagnosed?
Malaria can be diagnosed through various methods, depending on the availability of resources and healthcare settings.
Microscopic Blood Smears:
A small amount of the patient's blood is smeared on a glass slide, stained with special dyes, and examined under a microscope. Trained laboratory technicians can identify the presence of Plasmodium parasites and determine the species of the parasite.6
Rapid Diagnostic Tests:
RDTs are commonly used in areas with limited access to laboratory facilities. These are simple, portable test kits that detect specific malaria antigens in a patient's blood. They provide quick results, often within 15-20 minutes, and are especially useful in remote or resource-limited settings.6
Molecular Polymerase Chain Reaction (PCR) Tests:
PCR-based tests are highly sensitive and can detect very low levels of malaria parasites in a patient's blood. PCR tests are typically performed in a lab setting and results can take hours or days. They are particularly useful for research, surveillance, and in cases where a highly accurate diagnosis is required.6
Serological Tests:
Serological tests detect antibodies produced by the patient's immune system in response to a malaria infection. These tests are useful for epidemiological studies and can indicate past exposure to the parasite, but they are not typically used for diagnosing acute cases of malaria.6


How is Malaria Diagnosed?
Malaria can be diagnosed through various methods, depending on the availability of resources and healthcare settings.
Microscopic Blood Smears:
A small amount of the patient's blood is smeared on a glass slide, stained with special dyes, and examined under a microscope. Trained laboratory technicians can identify the presence of Plasmodium parasites and determine the species of the parasite.6
Rapid Diagnostic Tests:
RDTs are commonly used in areas with limited access to laboratory facilities. These are simple, portable test kits that detect specific malaria antigens in a patient's blood. They provide quick results, often within 15-20 minutes, and are especially useful in remote or resource-limited settings.6
Molecular Polymerase Chain Reaction (PCR) Tests:
PCR-based tests are highly sensitive and can detect very low levels of malaria parasites in a patient's blood. PCR tests are typically performed in a lab setting and results can take hours or days. They are particularly useful for research, surveillance, and in cases where a highly accurate diagnosis is required.6
Serological Tests:
Serological tests detect antibodies produced by the patient's immune system in response to a malaria infection. These tests are useful for epidemiological studies and can indicate past exposure to the parasite, but they are not typically used for diagnosing acute cases of malaria.6


The WHO Master Surveillance Protocol
In 2020, the World Health Organization (WHO) established a Master Surveillance Protocol for PFHRP2/3 Gene Deletions and Biobanking to support improved research and development of next generation testing technologies in malaria endemic countries.7
Primary Survey and Research Objectives:
The Surveillance Protocol was primarily created to determine whether the local prevalence of deletions in the PFHRP2/3 genes causing false-negative conventional RDT results among symptomatic patients has reached a threshold that might require a national change in malaria RDTs.
malaria by the numbers
94% of Malaria cases (233 million) occur in low-and-middle income African countries.1,8
Malaria causes the continent an estimated annual loss of $12 billion in direct costs.1,8
There were 249 million cases of malaria in 2022 (+5 million from 2021).1,8
Four African countries account for over half of all Malaria deaths worldwide.1,8



Nigeria - 31%
67+ million cases
188,480 deaths



Niger - 6%
7+ million cases
36,480 deaths



D.R. Congo - 12%
29+ million cases
72,960 deaths



U.R. Tanzania - 4%
67+ million cases
188,480 deaths
Learn More About How The WHO is Responding to This Challenge
The WHO Response plan to pfhrp2 gene deletions
Malaria diagnosis: Addressing the issue of HRP2 gene deletions
Resources
References
- World Health Organization. World Malaria Report 2023. https://www.who.int/publications/i/item/9789240086173 Accessed December 4, 2023.
- Jejaw Zeleke A, Hailu A, Bayih AG, et al. Plasmodium falciparum histidine-rich protein 2 and 3 genes deletion in global settings (2010–2021): a systematic review and meta-analysis. Malar J. 2022;21(1):26. doi:10.1186/s12936-022-04051-7.
- Prosser C, Gresty K, Ellis J, Meyer W, Anderson K, Lee R, Cheng Q. Plasmodium falciparum Histidine-Rich Protein 2 and 3 Gene Deletions in Strains from Nigeria, Sudan, and South Sudan. Emerg Infect Dis. 2021;27(2):471-479. doi:10.3201/eid2702.191410. PMID: 33496220; PMCID: PMC7853540.
- World Health Organization. Malaria Threats Map - Diagnosis Theme. https://apps.who.int/malaria/maps/threats/#/stories?theme=diagnosis. Accessed October 5, 2023.
- Iriart X, Menard S, Chauvin P, et al. Misdiagnosis of imported falciparum malaria from African areas due to an increased prevalence of pfhrp2/pfhrp3 gene deletion: the Djibouti case. Emerg Microbes Infect. 2020 Dec;9(1):1984-1987. doi: 10.1080/22221751.2020.1815590. PMID: 32869688; PMCID: PMC7534257.
- CDC. (2021). Malaria’s impact worldwide. Accessed October 5, 2023. https://www.cdc.gov/malaria/malaria_worldwide/impact.html.
- World Health Organization. Master protocol for surveillance of pfhrp2/3 deletions and biobanking to support future research. Accessed October 5, 2023. https://iris.who.int/bitstream/handle/10665/331197/9789240002050-eng.pdf.
- UNICEF Data: Malaria. https://data.unicef.org/topic/child-health/malaria/ Accessed November 21, 2023.




