GLOBAL POINT OF CARE
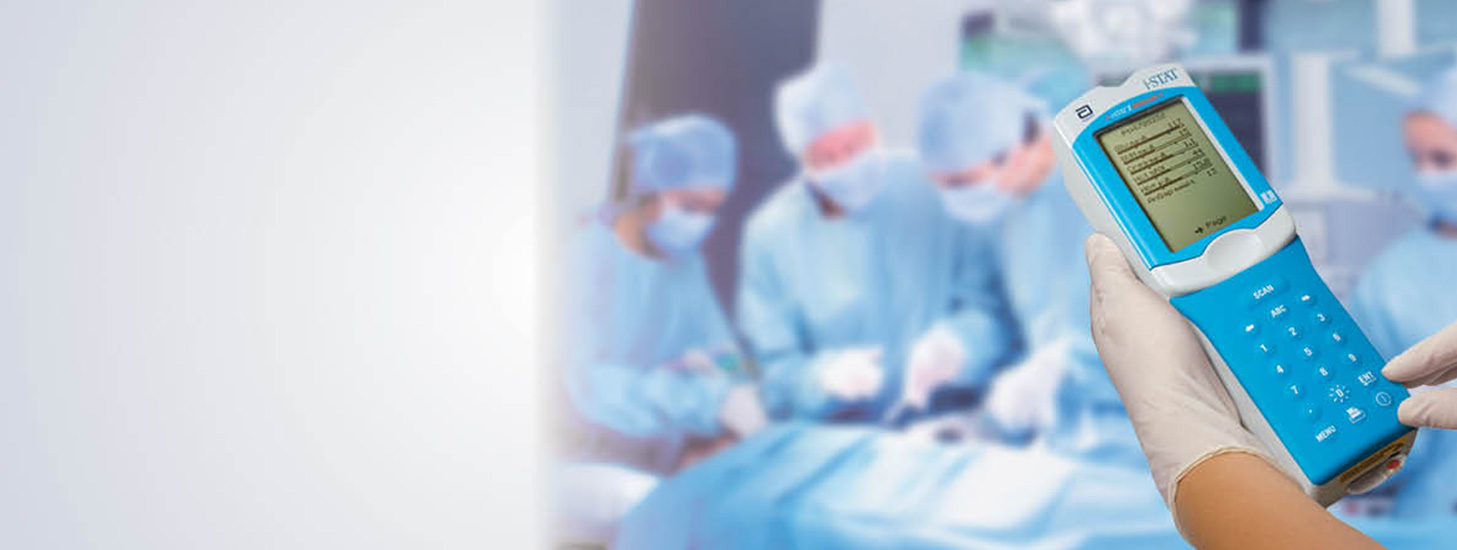
Abbott Point of Care (APOC) is committed to making a noticeable difference in people's lives across the world by:
- Improving the quality of care
- Increasing efficiency
- Reducing the total cost of care
As part of this commitment, APOC’s Investigator-Sponsored Studies (ISS) Program supports scientific research to enhance the understanding of our products, the potential applications and to explore unmet clinical needs. Support for approved proposals may be provided in the form of funding for study activities, products or both.
iss program mission
ISS program Mission
to support the advancement of scientific and clinical knowledge of apoc products in diverse clinical settings / therapeutic areas through independent original research.
The ISS Program is offered globally to all academic and community-based scientists and physicians interested in conducting research involving Abbott Point of Care products.
Areas of Interest
APOC has scientific interest in evidence that demonstrates in real-world settings:
CLINICAL IMPACT
The clinical impact of fast, accurate diagnosis using APOC products in various hospital and outpatient settings for different patient populations.
quality impact
The impact of APOC product implementation on the quality of care, including physician, caregiver and patient satisfaction.
economic impact
Economic benefits of APOC product implementation in different geographies and reimbursement / payment models.
process impact
Workflow improvements, operational efficiency gains, institution quality metrics enhancements with the implementation and standardization of APOC products.
investigator responsibilty



It is the responsibility of the qualified third-party investigator and investigator institution to develop, design and conduct the study in adherence with all ethical standards and country-specific regulatory requirements. Results of these studies are expected to be published in peer-reviewed medical journals and congresses for knowledge sharing across the medical community.
APOC does not assume any responsibility or liability for the study design, protocol development, initiation, conduct, data analysis, or reporting of results.
Additional investigator responsibilities, such as periodic updates to APOC Clinical Affairs and deliverables in the form of study reports and draft manuscript upon completion of study, etc. will be noted in the Research Study Agreement.
iss application process
the study application process is managed through the online ISS PORTAL
We cannot accept requests via e-mail or other means of communication. ISS applications are currently being accepted on a rolling submission basis.
this portal is not to be used for donation or charitable contributions. for such requests, please work with your local apoc liaison.
create an account on the iss portal
When you are ready to submit your application, visit the ISS portal and create a profile to initiate the process and to gain access to the concept or proposal form (see Submission Requirements below).
Prepare your submission
As an investigator, you have the option to initiate an application by either submitting a brief study concept or a full proposal. If you submit a concept and it is approved, you will be asked to submit a full proposal, including study protocol, timeline, and publication plan.
SUBMISSION REQUIREMENTS
- Well-written, completed ISS concept or proposal form (in English only)
- Investigator Curriculum Vitae (signed and dated within last two years)
- Itemized budget when requesting funding or study products
Application review
All applications are evaluated by our cross-functional Scientific Review Committee (SRC), comprised of members from Clinical, Medical & Regulatory Affairs, Research & Development, and other functions, as appropriate. Applications are evaluated based on predefined criteria, such as scientific merit of research, alignment with our areas of strategic interest, investigator research experience, available funding, etc. As such, submission of a proposal does not guarantee approval.
Application status / Notification
You may check the status of your application at any time, by visiting the ISS portal. If your proposal is approved, APOC will notify you via e-mail and ask you to submit additional study documents for final review and/or to initiate a Research Study Agreement. Funding and/or product support may be released only after full execution of the Research Study Agreement, in accordance with the terms and milestones set forth.
FAQs
what does “investigator-sponsored” mean? isn’t abbott point of care the sponsor?
An investigator-sponsor is an individual or institution responsible for fulfilling the obligations of both a sponsor and investigator. As the sponsor, the investigator assumes all responsibilities related to applicable regulatory requirements and ethical standards and ensures good clinical practice in the conducting of the study. As the investigator, he/she is responsible for the design, development, and conduct of the study from initiation to close-out. Abbott Point of Care is not the sponsor for this type of study and holds no responsibility or liability for the study conduct, data analysis, or reporting of results.
how do i submit a proposal?
To submit a proposal, please visit the ISS portal and create a profile to initiate the process.
what types of support can be requested?
Support may be requested in the form of products (i-STAT analyzer as loaner for duration of study, single-use test cartridges and other supplies), and/or funding for study-related activities. When requesting funding, the budget must be clearly defined in the application and reflect ‘fair market value’ for all costs. APOC cannot make payments to the investigator or institution as author honoraria.
how long does it take to review the request?
The SRC review process strives to provide timely responses to all applications. Status of each application can be viewed at any time by visiting the ISS portal My Submissions tab. Because applications are reviewed on a rolling basis, specific timings vary on numerous factors, including volume of requests received, nature of proposal, etc.
how can i check the status of my application?
Please visit ISS portal, login to your profile and view the status of your application under the Submission tab.
How Is APOC Involved Through The Course Of An Investigator-Sponsored Study?
As outlined in executed Research Study Agreement, APOC will expect periodic progress reports through the duration of the study to be submitted through the ISS portal. Reports will be used as documentation to determine if study milestones have been met and/or product shipping requests can be fulfilled. Study payments will be made only upon documented milestone achievement.
My question wasn't answered, who can I contact?
Please send an e-mail inquiry with your question and someone from the Abbott Point of Care Clinical Affairs team will be in touch in 1-2 business days.


