GLOBAL POINT OF CARE
a leader in rapid point-of-care diagnostics. answers in your hands.
Point-of-care diagnostic testing provides immediate, actionable results, right where you need them. The time has come to bring the healthcare to the people. Get immediate answers to make better diagnostic decisions in the moment.
Panbio™ COVID-19 Antigen Self-Test
(NEW) Panbio™ COVID-19 Antigen Self-Test is simple solution for COVID-19 infection detection, with rapid results in the convenience of your home.
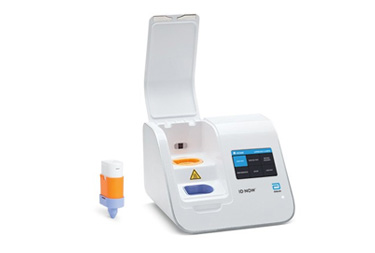
ID NOW™ COVID-19
The ID NOW™ COVID-19 assay is available for use on the ID NOW platform under U.S. Food and Drug Administration Emergency Use Authorization (EUA). The ID NOW™ COVID-19 rapid test delivers high-quality molecular positive results in as little as 5 minutes, targeting the coronavirus (COVID-19) RdRp Gene.
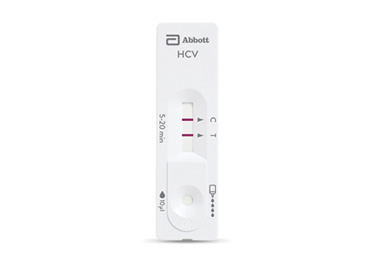
Bioline™ HCV
Bioline™ HCV test is a immunochromatographic rapid test for the qualitative detection of antibodies specific to HCV in human serum, plasma or whole blood. When speed matters, choosing a highly sensitive rapid HCV test with a safe fingerstick procedure can make a difference.
Bioline™ H.pylori
Bioline™ H.pylori test is a rapid test for the qualitative detection of antibodies of all isotypes (IgG, IgM, IgA) specific to Helicobacter pylori in human serum or plasma.
Determine™ HBsAg 2
With an analytical sensitivity of just 0.1 IU/mL, Determine™ HBsAg 2 is a highly sensitive, easy to use rapid lateral flow test that enables quick, accurate identification of HBsAg positive patients, increasing HBV case finding and facilitating fast and appropriate linkage to care in any healthcare setting.
i-STAT Family of products - DIAGNOSE WITH CONFIDENCE
i-STAT POCT solutions is an easy, integrated point-of-care testing (POCT) solution for a broad range of clinical settings including the TBI Plasma Test, a biomarker-based assay designed to objectively assess the need for CT scan in suspected TBI cases.
i-STAT 1
Lightweight, portable and easy to use, the i-STAT 1 blood analyzer operates with the advanced technology of i-STAT test cartridges. Together, they create the i-STAT System - a point-of-care testing platform that provides healthcare professionals with diagnostic information when and where it's needed.
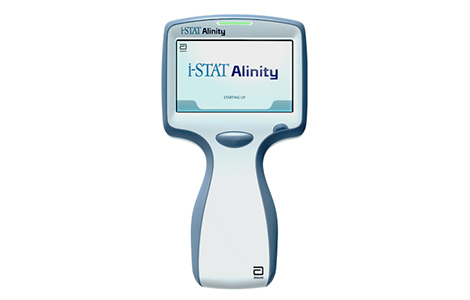
i-STAT Alinity
The i-STAT Alinity was built on the proven technology of the i-STAT 1 System, and is an easy-in-use, portable bloody analyzer that delivers real-time, lab-quality diagnostic test results. The i-STAT Alinity's award-winning design features a more intuitive interface that simplifies the testing process even further, allowing for minimal operator training. i-STAT Alinity is the i-STAT family's most advanced testing solution.
SIMPLY MORE EFFICIENT
Abbott is transforming care by giving people – and their doctors – timely information to better manage health. We offer chronic care management testing in multiple settings, and CRP point of care testing to support the reduction of antimicrobial resistance. Our products provide highly accurate test results during the patient consultation, using only a tiny fingerstick or urine sample.
AFINION™ 2 System
The AFINION™ 2 System is a global market leader in highly accurate point-of-care (POC) testing for physician offices and clinics. The Afinion™ 2 analyzer is a compact, rapid, multi-assay analyzer that provides valuable near patient testing at the point-of-care.
The NycoCard™ CRP
The NycoCard™ CRP is an immunochemical assay for the quantitative determination of C-reactive protein (CRP) in whole blood, serum and plasma. The measurement of CRP provides information for the detection and evaluation of infection, inflammatory disorders and associated diseases.
coprehensive answers when and where they are needed most
Our rapid drug screening devices offer high quality, convenient options as well as complete on-site drug testing confidence. Now you can equip yourself with dependable, real-time screening results.
The SoToxa™ Mobile Test System
The SoToxa™ Mobile Test System is designed for rapid drug screening and detection in oral fluid. With test results in minutes, this handheld analyzer is lightweight, compact, and easy to use
POCT DATA CONNECTIVITY
Across the hospital, from campus to campus or from one state/province to another, our web-based connectivity data management platforms unlocks efficiency in point-of-care testing program management.