Global Point of Care



TEST NOW. TREAT EARLIER. GET WELL SOONER.
If you or someone you care about has symptoms like a cough, congestion, or a sore throat, it can be confusing to know what is brewing. In just minutes, rapid testing can help pinpoint what’s behind those symptoms so your clinician can recommend the right treatment to target the specific illness.
Find a healthcare professional near you to get tested or buy a self test in a store or online.
RAPID TESTING CAN IDENTIFY RESPIRATORY ILLNESS QUICKLY
You need answers. There’s no substitute for knowing exactly what’s causing you or someone you care about to feel sick. That knowledge will help you get the right treatment for a quicker recovery.
Rapid testing can identify the illness driving symptoms—and it takes just minutes to get the result. A healthcare professional can order a rapid molecular test, which you can have performed at a pharmacy, clinic, or other convenient care location. Or you can buy a self test for at-home testing. Both rapid testing options can help speed up recovery.
Rapid testing provides an earlier diagnosis, potentially avoiding complications from various respiratory illnesses.2-4 And with an early, correct diagnosis you can take the right precautions to keep from spreading your illness to loved ones.
Find the right test for you.
Rapid tests are essential tools to help your clinician diagnose what’s behind your symptoms, so you can treat earlier and get well sooner.


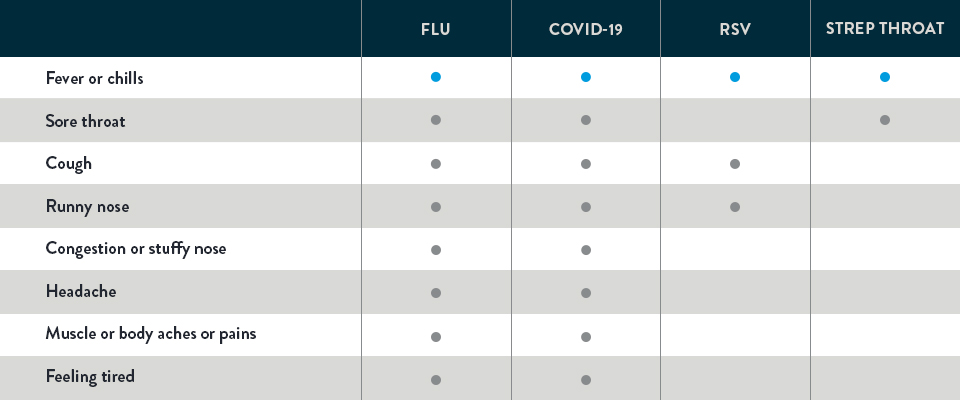
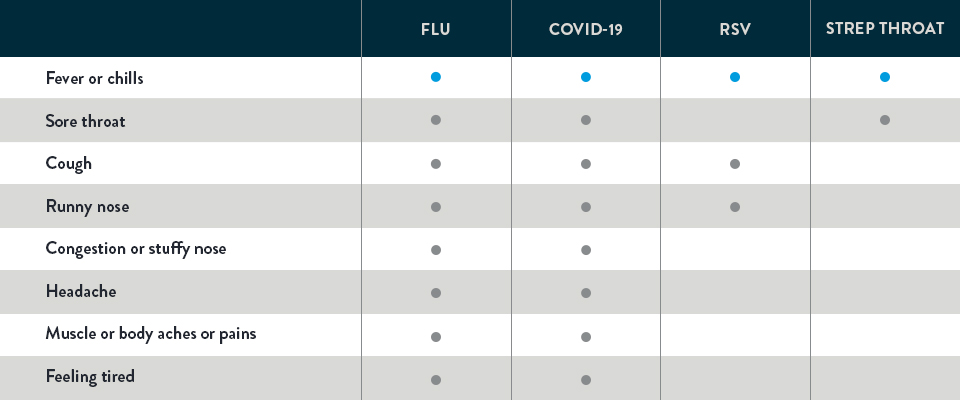
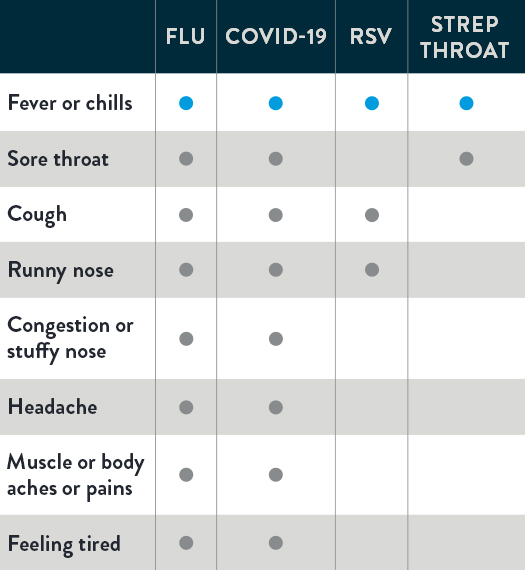
OVERLAPPING COMMON SYMPTOMS OF THE FLU, COVID-19, RSV, AND STREP THROAT1-3
Take a look. Below you can see that flu, COVID-19, RSV, and strep throat share similar symptoms. But different illnesses call for different treatments. As you can see, symptoms alone can’t determine the specific illness. It takes testing to help provide the key to what’s wrong so the appropriate treatment can be recommended—and help you feel better faster.
Experiencing symptoms?
Learn more about rapid testing.
HERE’S WHY TESTING IS SO IMPORTANT
If you or someone you care about has respiratory symptoms—coughing, sneezing, or a sore throat for example—it’s tempting to assume it’s a cold or the flu. But you could be wrong. Other possibilities include COVID-19 or RSV or step throat. And how each is treated could make all the difference in recovering—and recovering without complications.
Rapid testing is available for flu, COVID-19, RSV, AND strep throat. Here’s what you should know:

Flu
Flu is not only no fun to experience, but it’s contagious. Getting tested with a rapid flu test can help you get the appropriate treatment. Not only will that help you feel better sooner but it can also keep you from spreading the flu to family and the community.5,6

COVID-19
Keep yourself and those that you’re in contact with safe by taking a rapid COVID-19 test as soon as symptoms show up. It can help you make informed decisions about treatment.7

RSV
RSV stands for Respiratory Syncytial Virus. By taking a rapid syncytial virus test, you may feel relief that your cold-like symptoms are caused by a virus that tends to run its course without requiring treatment.3,6

Strep Throat
The faster you know if strep throat is causing symptoms, the sooner your clinician can determine if treatment includes antibiotics. That’s why a rapid strep test is so useful. You’ll get the answer in minutes, and that could also eliminate the need for a follow-up throat culture.


HERE’S WHY TESTING IS SO IMPORTANT
If you or someone you care about has respiratory symptoms—coughing, sneezing, or a sore throat for example—it’s tempting to assume it’s a cold or the flu. But you could be wrong. Other possibilities include COVID-19 or RSV or step throat. And how each is treated could make all the difference in recovering—and recovering without complications.
Rapid testing is available for flu, COVID-19, RSV, AND strep throat. Here’s what you should know:

Flu
Flu is not only no fun to experience, but it’s contagious. Getting tested with a rapid flu test can help you get the appropriate treatment. Not only will that help you feel better sooner but it can also keep you from spreading the flu to family and the community.5,6

COVID-19
Keep yourself and those that you’re in contact with safe by taking a rapid COVID-19 test as soon as symptoms show up. It can help you make informed decisions about treatment.7

RSV
RSV stands for Respiratory Syncytial Virus. By taking a rapid syncytial virus test, you may feel relief that your cold-like symptoms are caused by a virus that tends to run its course without requiring treatment.3,6

Strep Throat
The faster you know if strep throat is causing symptoms, the sooner your clinician can determine if treatment includes antibiotics. That’s why a rapid strep test is so useful. You’ll get the answer in minutes, and that could also eliminate the need for a follow-up throat culture.


read more about rapid testing

BINAXNOW™. KNOW NOW.
During the early stages of the pandemic, we helped pioneer the technology used on the frontlines to test for COVID-19. Now that same technology is available to you in a convenient at-home self test. You can choose between testing for COVID-19 only or COVID-19 and Flu combined.



Discover ID NOW™ rapid molecular testing
ID NOWTM rapid molecular instrument provides your healthcare professional with timely results, that can inform your treatment, which may include antivirals or antibiotics, so you can get well sooner.

FIND THE BEST TEST FOR YOU
Rapid tests are essential tools to help diagnose what’s behind your symptoms, so you can treat earlier and get well sooner. Understand the differences between rapid molecular tests and at home COVID-19 antigen self tests.
REFERENCES
- "Symptoms of COVID-19." Centers for Disease Control and Prevention (CDC), last updated February 22, 2021. https://www.cdc.gov/coronavirus/2019-ncov/symptomstesting/symptoms.html.
- "Flu Symptoms & Complications." Centers for Disease Control and Prevention (CDC), last reviewed September 21, 2021. https://www.cdc.gov/flu/symptoms/symptoms.htm.
- "Symptoms and care of RSV (respiratory syncytial virus)." Centers for Disease Control and Prevention (CDC), last reviewed September 24, 2021.
- "Strep throat: all you need to know." Centers for Disease Control and Prevention (CDC), last reviewed January 12, 2021. https://www.cdc.gov/groupastrep/diseases-public/strep-throat.html.
- Uyeki, Timothy M., et al. “Clinical Practice Guidelines by the Infectious Diseases Society of America: 2018 Update on Diagnosis, Treatment, Chemoprophylaxis, and Institutional Outbreak Management of Seasonal Influenza.” Clinical Infectious Diseases 68, no. 6 (March 15, 2019): e1-e47. https://doi.org/10.1093/cid/ciy866.
- Azar, Marwan M., Marie L. Landry. “Detection of Influenza A and B Viruses and Respiratory Syncytial Virus by Use of Clinical Laboratory Improvement Amendments of 1988 (CLIA)-Waived Point-of-Care Assays: A Paradigm Shift to Molecular Tests.” Journal of Clinical Microbiology 56, no. 7 (July 2018): e00367-18. https://doi.org/10.1128/JCM.00367-18.
- “COVID-19 Testing: What you need to know” Centers for Disease Control and Prevention (CDC), last reviewed May 11, 2023 https://www.cdc.gov/coronavirus/2019-ncov/symptoms-testing/testing.html.



